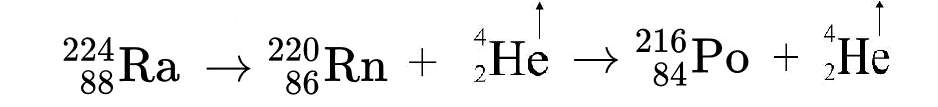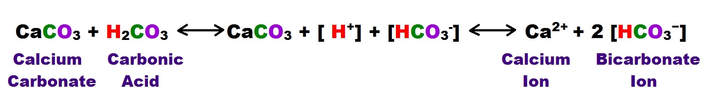The Karst Page
The first three tabs below outline the general aspects of karst features for those who may be new to caving, and includes quite a few examples and diagrams, from both here in the hills and elsewhere. The next two tabs go on to surmise my current thoughts on Black Hills area caves and their speleogenesis while the last tab deals with the geochemical aspects of cave formation. There is a lot more science and opinion in the last three tabs, but that’s a natural consequence of my background, many years of observations and the countless discussions I’ve shared with so many like-minded and observant souls.
No apologizes for the opinions, as I hold that I come by them honestly. We should each openly embrace the freedom to question and challenge…that’s what science, learning and the pursuit of knowledge are all about.
Keep pushing the envelope or you’ll never get out of the bubble of complacency.
KME
“Consider your origin. You were not formed to live like brutes but to follow virtue and knowledge.”
― Dante Alighieri, The Divine Comedy
Karst Definition
Karst is a distinctive type of topography and morphology formed from the dissolution of soluble rocks such as limestone, dolomite and gypsum. Karst areas are typically characterized by underground drainage systems with sinkholes, dolines and blind valleys on the surface and by caves, solution cavities and solution enlarged fracture systems in the subsurface.
- Karst features can develop both above and below the water table and they are typically classified based on that distinction.
- The karst features that develop below the water table are referred to as having a “phreatic” origin and those that take place above the water table are due to”vadose” processes.
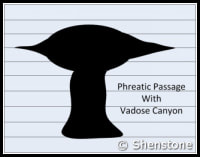
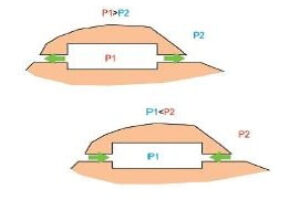
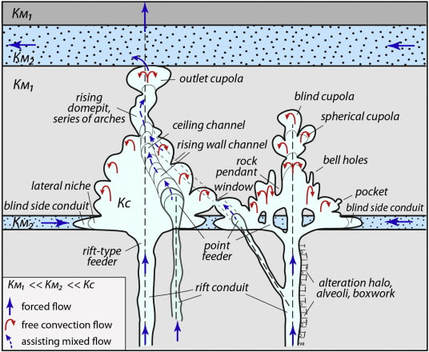
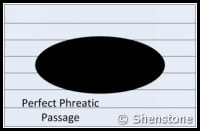
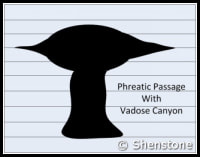
- Phreatic passages are wider than they are tall. They tend to meander and have rounded or flattened ovoid profiles as shown in the adjaent diagram. Phreatic passageways are typically found developed along bedding planes.
- Vadose passages and developments tend to be linear, and follow fracture systems or to be modifications of earlier phreatic features, In general, vadose developments tend to be taller than they are wide and often have a canyon-like notch at the base of the passage.
- Since vadose modifications often account for the later-stage decorative formations overprinted on earlier phreatic features, vadose developments are sometimes referred to as “secondary” and the phreatic type as “primary”.
- Karstic and cave systems can develop almost entirely from one or the other of these two end-member processes, but most have origins due to recognizable contributions from both. The adjacent diagram shows a phreatic passage that has been modified by a vadose notch or “canyon” at the base.
- There are many terms used in various parts of the world for karst related features. The usages are sometime overlapping, and can be confusing depending on the areas they describe. For a more complete listing and decoding of karst terms, it is helpful to refer to one of the the following sources:
Typical Surface Karst Features
Sinkholes & Dolines
- Sinkholes and dolines are both quite common in most karstic terrains, but are not quite as widespread in the Black Hills as in other major karst areas. Nonetheless, they are a locally prominent feature in some of our better explored areas of Karst development.
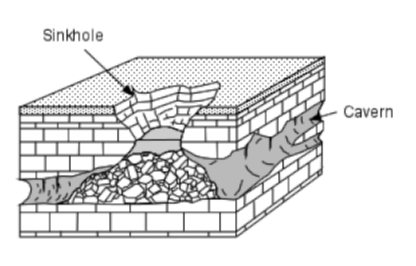
- Sinkholes are steep sided, roughly circular collapse features which often lead directly or indirectly into an underground cavity. The adjacent diagram shows what a fully developed sinkhole collapse looks like.
- Although numerous Black Hills caves have sinkhole entrances, many more are accessed through enlarged solution cavities and fractures exposed in drainages, bluffs and cliffs.
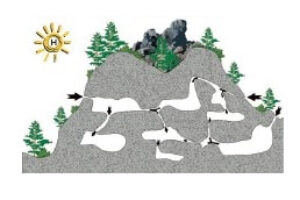
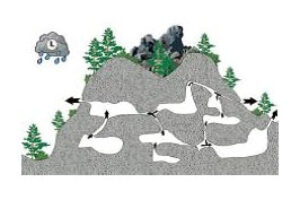
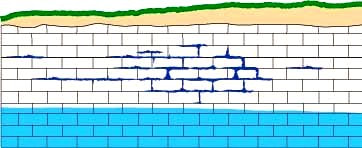
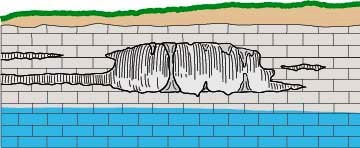
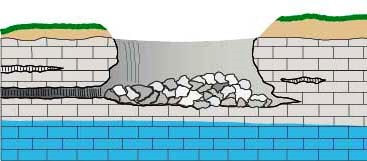
- The following panel of diagrams show the development cycle of a typical sinkhole from incipient dissolution, through cavern enlargement and then through eventual collapse. In the instance shown below, all of the cavern growth takes place in the vadose zone above the water table, which is less common here in the Black Hills than in some other areas.
- A doline is a shallow funnel or dish-shaped depression due to the slow dissolution of bedrock where there are intersecting networks of fissures and fractures.
- With time, a doline may develop into a sinkhole, especially where there is a corresponding solution cavity of sufficient size directly below the feature.
- Various dolines are illustrated in the diagram below with the middle feature transitioning into a sinkhole. As has been suggested in the descriptions above, there is a complete continuum between dolines and sinkholes and in some areas, these terms are used interchangeably.
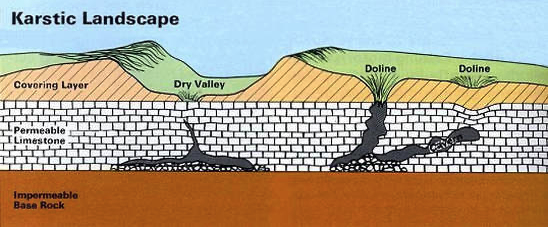
- Some dolines here in the hills contain shallow ephemeral ponds and wetlands that hold rainwater for extended periods until it seeps downwards. There are other examples, however, that remain filled with water and do not dry up. These features are likely spring fed.
- Dry Lake (ephemeral) and Red Lake (perennial) in the northern part of the Black Hills are both examples of dolines but there are many others throughout the region.
Epikarst
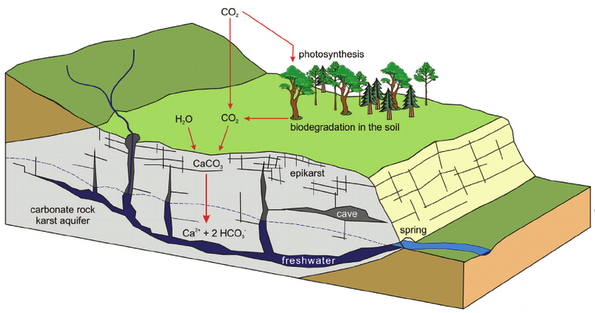
- Epikarst refers to the features found in the upper part of a karst system, in which water is stored and modified before it percolates into underlying aquifers.
- Epikarst includes small solution pockets , minor solution enlarged fractures, small fissures and residual soils that form in and over soluble bedrock.
- These feature capture and hold surface water long enough to allow it to saturate with atmospheric carbon dioxide and the organic acids in the soil, thus forming a more efficient acidic solution for dissolving rocks at depth.
- Physical expressions of epikarst are subtle, but may include spongy appearing outcrop exposures, small divot-like depressions in the soil and faintly elongated fracture and fissure trends in bedrock and within their thin overlying soils.
- The reddish residual soils known as Terra Rosa that form over carbonate bedrocks can also be considered as epikarstic, as they are clay rich and hold water for extended periods of time.
Lapies (Karren) & Grikes
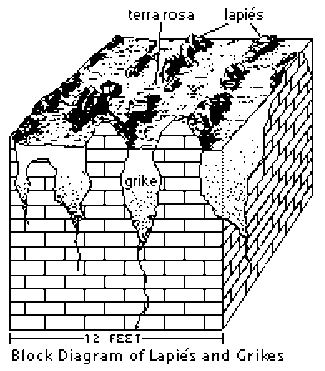
- Lapies (karren) and grikes are near surface features that result from dissolution of soluble limestone and gypsum bedrock along parallel fractures.
- These features are most typically observed in areas with deeply weathered carbonate bedrock. In the Black Hills, these features are more restricted than in other karst terrains, as the climate, on the whole, is semi-arid. On moist north and northeast facing slopes in the higher altitude areas, however, they can be observed.
- The diagram illustrates the typical geometry and scale of lapies and grikes.
- Grikes are solution widened and clay/debris filled fractures systems found in weathered limestone areas that are separated from one another by narrow ribs of relatively unweathered residual bedrock known as lapies or karren.
- The terms lapies and karren are used interchangeably in most areas, with karren being the German/European equivalent of the other.
- These features often result in a series of low, parallel and closely spaced ribs of rock separated by shallow soil filled depressions. Lapies and grikes can be seen clearly exposed in road cuts in the areas where they occur.
- The reddish, clay rich soil that fill the grikes is known as Terra Rosa. This soil type is a distinctive weathering feature in karst terrains worldwide. It is found in moist areas where outcrops are sparse and weathering has created continuous soil cover over the underlying carbonate rocks.
Lapies (Karren) & Grikes
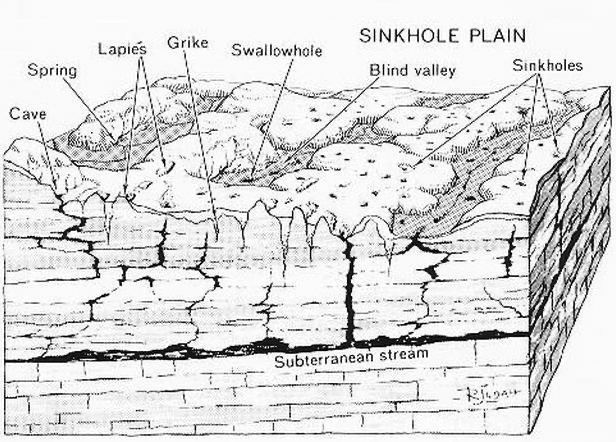
All of the features described above are common in well developed karst terrains.
Many other unusual and characteristic features can also be observed in karst areas. Some of these are shown on the diagram above. They include:
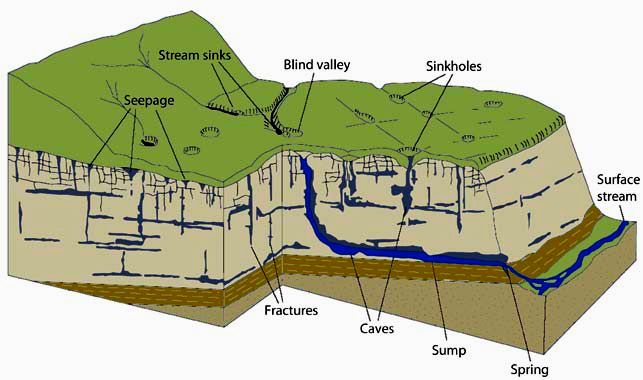
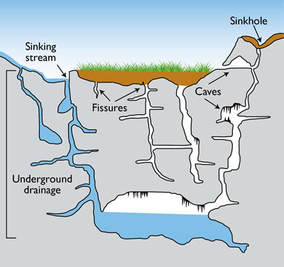
- Stream Sink or Capture Zone – A zone along a drainage where water flow infiltrates into the stream bed over a short distance.
- Swallow Hole – A place where a stream disappears abruptly into a hole or whirlpool.
- Subterranean Stream – Streams found in partially or completely flooded passages that are fed through underground springs and/or from water inflow from the surface.
- Blind Valley – A drainage where the collapse of a cave system has led to a valley shaped depression with no outlet on one end.
Typical Subsurface Karst Features
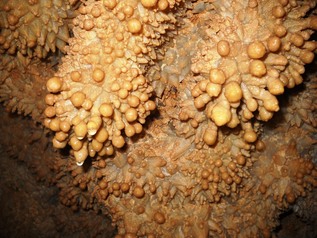
Here are just a few of the subsurface karstic features seen in Black Hill caves with their generally accepted definitions and a few local photographs illuminating their characteristics. Click on the images for a closer look.

Stalactites and Stalagmites
- ARAGONITE – A relatively uncommon crystalline form of calcium carbonate (CaC03) that is denser than calcite and which has an orthorhombic crystal structure. It is one of the principal minerals in frostwork and precipitates through evaporation as solutions become calcium depleted and magnesium enriched.
- BOXWORK – A type of mineral structure formed by erosion/dissolution rather than accretion, sometime found in caves and erosive environments. Typically consists of a network of thin blades of calcite or gypsum etched out in relief on the limestone walls and ceiling of a cave.
- BREAKDOWN – Fall of rock from the roof or wall of a cave
- CALCITE – The most common of the calcium carbonate (CaC03) minerals and the main constituent of limestone. it has several different crystal forms, all within the rhombohedral subsystem.
- COLUMN – A speleothem from floor to ceiling, formed by the growth and joining of a stalactite and a stalagmite, or by the growth of either to meet bedrock.
- DOG-TOOTH SPAR – A variety of calcite with acute-pointed crystals, commonly seen as overgrowths on surfaces. This mineral form typically precipitates below the water table and is phreatic in origin. when the tips of the crystals are not pointed but rather a flat surface, they are called “Nail-Head” spar
- DOME – A large hemispheroidal hollow in the roof of a cave, formed by breakdown, dissolution or weathering, generally in mechanically weak rocks, which prevents bedding and joints from dominating the form.
- DRIPSTONE – Calcium carbonate deposited from water dripping from the ceiling or wall of a cave or from the overhanging edge of a rock shelter; commonly refers to the rock in stalactites, stalagmites, and other similar speleothems; in some places composed of aragonite or gypsum.
- DRIPSTONE CURTAIN – A speleothem in the form of a ribbon or folded sheet, often translucent and resonant, hanging from the roof or wall of a cave.
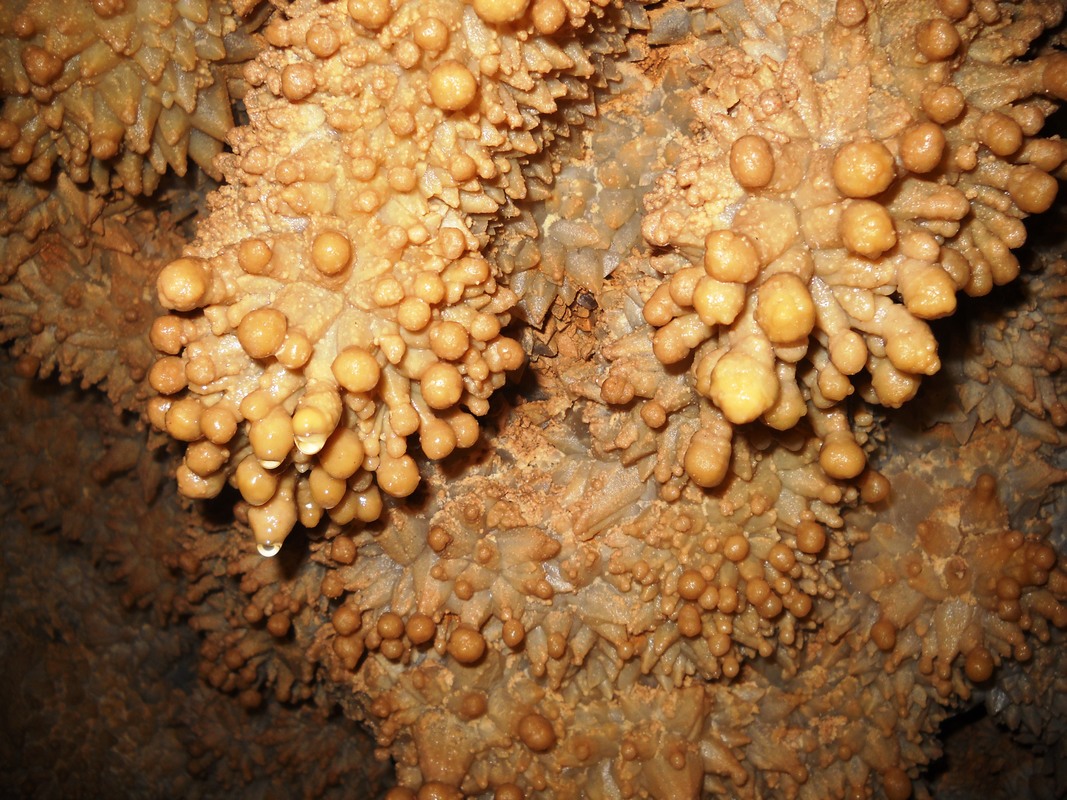
- FLOWERS – (or Cave Flowers) A speleothem composed of long needle-like crystals situated in clusters which radiate outward from a common base. The “needles” may be quill-like or feathery. Most are made of the mineral aragonite (Ca·Mg(C03)2), although some noteworthy examples are composed of gypsum (CaSO4·2H2O). These features are more properly termed “Anthodites”.
- FLOWSTONE – Deposits of very thin films of calcium carbonate, gypsum, and other mineral matter which have accumulated on the walls or floors of caves at places where water trickles or flows over the rock.
- FROSTWORK – a type of speleothem with acicular (“needle-like”) growths almost always composed of aragonite or calcite replaced by aragonite. A type of anthodite (see Flowers above). In some caves frostwork may grow on top of cave popcorn or boxwork.
- GROTTO – A room in a cave of moderate dimensions but richly decorated. Also refers to an NSS chapter.
- GYPSUM – The mineral hydrated calcium sulfate (CaSO4·2H2O) which is very soft and can sometime crystallize in flower-like growths (called anthodites or flowers).
- HELICTITE – A speleothem, which at one or more stages of its growth changes its axis from the vertical to give a curving or angular form. Composed of a curved or angular twig-like lateral projection of calcium carbonate having a tiny central canal. Usually very delicate and generally quite rare.
- LOGOMITE – A hollow, rough surfaced stalagmite of problematic origin. They have the appearance of a volcanic spatter cone, and may be due to the splashing of sediment laden solutions during speleothem formation.
- PHREATIC ZONE – The zone where voids in the rock are completely filled with water below the water table, in which water moves under a hydraulic gradient in response to a hydrostatic head.
- POPCORN – Cave popcorn, or coralloids, are small nodes of calcite, aragonite or gypsum that form on surfaces in limestone caves. They are one of the commonest types of speleothem.
- RIMSTONE – A deposit formed by precipitation from water flowing over the rim of a pool.
- SODA STRAW– A long, thin-walled tubular stalactite less than about 1 cm in diameter. Generally very fragile and found to a greater or lesser extent in most Black Hills caves.
- SPELEOTHEM – A general term for cave formations, such as stalactites and stalagmites, which are secondary mineral deposits formed in a cave. Most of these features form above the water table and are considered to be vadose in origin.
- STALACTITE – A speleothem hanging downwards from a roof or wall, of cylindrical or conical form, usually with a central hollow tube.
- STALAGMITE – A speleothem projecting vertically upwards from a cave floor and formed by precipitation from drips.
- VADOSE ZONE – The zone where voids in the rock are partly filled with air and through which water descends under gravity.
The labelled Photograph below is from Cave photographer Dave Bunnell’s Under Earth Images Wikimedia Commons site, which shows most of the other major speleothems in a superbly decorated chamber. The photograph is not from a Black Hills cave but is too good to pass up sharing.
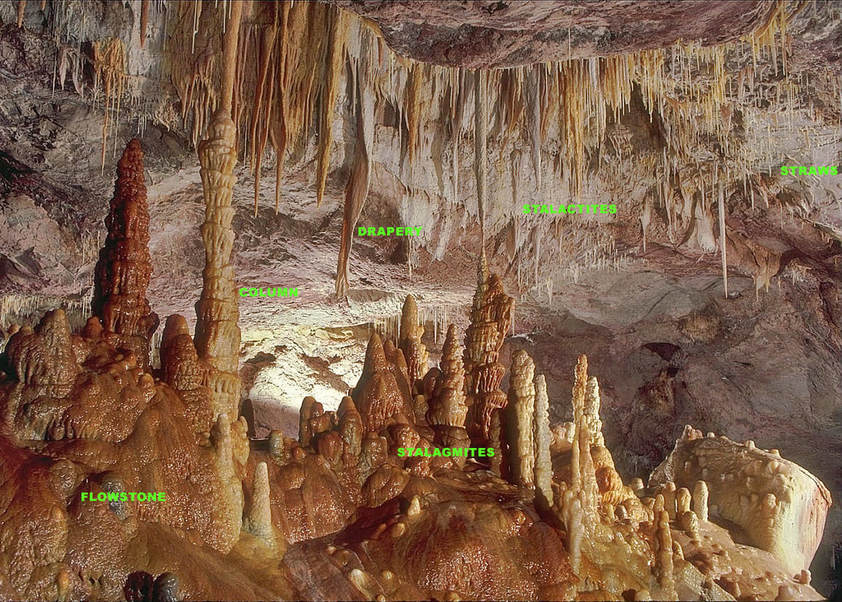
The Principal Cave & Karst bearing formation in the Black Hills, and the one that will be primarily described and discussed in the following section is the:
Pahasapa (Madison) Limestone
Areas of karst development are widespread in the overlying Minnelusa, Minnekahta and Spearfish Formations, however. Although caves do occur in all of these formations, the largest and most complexly developed systems are found within the Pahasapa Limestone and that is the formation upon which the majority of current speleological study and exploration is being centered .
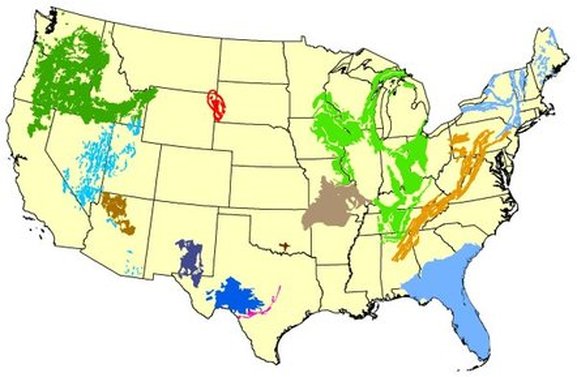
The Madison group, whose Black Hills outcrop pattern is shown in red on the adjacent map, forms part of the nations largest confined aquifer and is far & away one of the most distinctive and unusual karst aquifers in North America. It covers large portions of Montana, Wyoming, Southern Saskatchewan and North and South Dakota.
- The Pahasapa and upper Englewood Limestones form the Madison Group within the Black Hills uplift area.
- The Madison Group outcrops as a nearly continuous ring around the Black Hills uplift, as can be seen on the above map and the maps that follow.
- This formation is locally 300 to 600 ft. thick, and composed of massively bedded, cliff forming limestone and dolomite that is notably flinty and cavernous in it’s upper portions.
- This highly karstic formation has localized stream sinks on most every drainage that it crosses on it’s journey outwards from central part of the uplift. As such, the Black Hills constitutes one of the principal areas of recharge for this highly important and heavily utilized aquifer. The need to study, understand and protect this critical water bearing resource cannot be overstated.
- Most of the Pahasapa Limestone’s karst features are found in the formation’s upper one half to two thirds and nearest to it’s contact with the overlying Minnelusa Formation.
- Karstic features have been recognized in this same horizon in deep drill holes well out into the surrounding plains, which suggest that there was at least one other period of karstic development prior to the the most recent period. The high permeability and transmissivity of the zone outside of the zone where active speleogenesis might be expected also argues for an earlier episode, as well.
- Although parts of the Minnelusa Formation are also an aquifer, on the whole the formation acts as an effective barrier to vertical water flow into the underlying limestones. It is, in hydrological terminology, an aquaclude, and limits water inflow to the Madison except in those areas where it is not covered by the formation.
Picture
The pattern of the Madison Group’s outcrops are shown in the sky-blue pattern on the geologic map of the Black Hills Uplift shown in the diagram below.
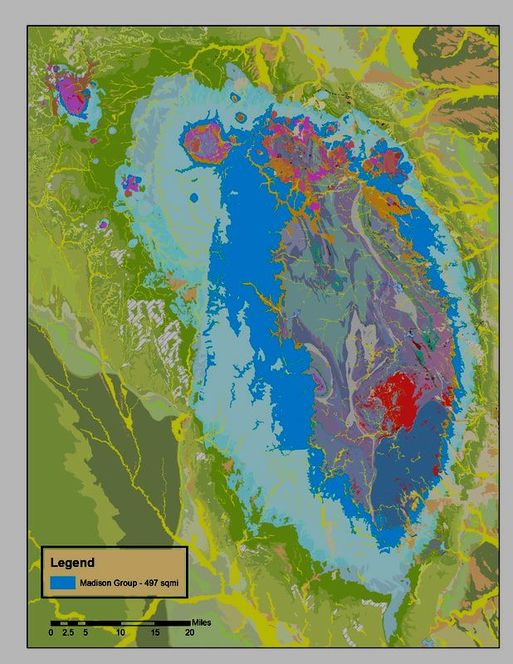
- It’s outcrop pattern covers almost 500 square miles of Black Hill’s terrain.
- The narrow nature of the exposures along the east and north sides of the Hills is due to the steep dip of the beds in these areas. There are also numerous prominent faults and folds in these steeply dipping areas.
- The broader pattern of Madison outcrops along the southern and western sides of the uplift are due to the opposite situation.
- In these areas, the gentler dipping beds are modified by fewer faults and folds, although some of these structural features are locally quite prominently developed.
- Many of the larger caves in the Black Hills are found in close proximity to such major structures and it can and often has been argued that there is a genetic relationship between the two.
This is most particularly the case with the larger Labyrinth style systems like Jewel, Wind, Stagebarn, Reed’s and Bethlehem caves.
Directly overlying the Madison group limestones and dolomites is a broad outcrop area of Minnelusa Formation sediments shown in light blue. This unit is a mix of sandstone, siltstone, shale and limestone and although it does not contain numerous caves, it does display widespread development of karst features. This is particularly the case within it’s basal portion.
- The Minnelusa Formation contains varying amounts of gypsum, and dissolution of that mineral has led to widespread karst collapses and other such features within the unit.
- Karstic features in the Minnelusa are often (but not always) an indicator of major dissolution in the underlying Madison Group, though the patterns of interrelationship can be quite complex and difficult to unravel.
- Minnelusa derived karstic fill is not infrequently found in the caves of the underlying Madison carbonates, particularity where they are located just below the unit’s upper contact.
The Minnekahta and Spearfish Formations are typically exposed at the outermost edges of the central Black Hills uplift. They are shown on the map above as the peripheral rings of highlighted bright green and darkest green respectively. Both of these formations locally host karst features, but they are particularly well developed within the Spearfish Formation, which contains thick bands of gypsum.
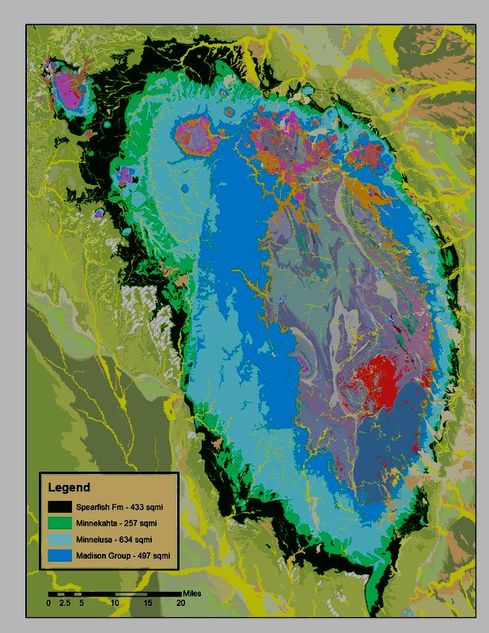
- Caves within the Minnekahta tend to be smaller and restricted to a relatively planar geometry, as the unit is at most 60′ thick, but they have many interesting features and have been found throughout the Hills region.
- The Spearfish Formation contains several thick layers of gypsum and has stringers of the same throughout its’ 400′ thickness. Gypsum’s high solubility has resulted in some fairly major and impressive sinkholes, dolines, collapse features and caves. Some of these features are expressions of long term ongoing solution in the underlying formations working its way upward, and others are the result of near surface processes more closely tied to climatic conditions.
- Although there are many caves within the Spearfish Formation, most of the discussions about karst features that follow are restricted to those within the Madison Group carbonates, as that is where the bulk of present day studies are taking place.
- At some later date, hopefully, a section dealing specifically with the gypsum caves in the Black Hills uplift will be added to this group of pages.
So much to do..so little time
“No thief, however skillful, can rob one of knowledge, and that is why knowledge is the best and safest treasure to acquire.”
―L. Frank Baum, The Lost Princess of Oz
“The more I learn, the more I realize how little I actually know”
(paraphrased from German)
Albert Einstein
This section is basically my geological mantra (some would say rant) surmised from reasonably detailed observations over the years and reliable geochemical information, but certainly not the final word on this subject. Just an educated guess from a decidedly opinionated guy.
“You can’t depend on your eyes when your imagination is out of focus.”
Samuel Clemens-Mark Twain
The Black Hills uplift contains a truly world class karstic terrain, which boasts the second and sixth largest caves on the planet as well as numerous other large and complex caves.
The karst features of this area are decidedly unique, however, in that they typically represent one end member of the hypogene (phreatic) to supergene (vadose) origin cycle for caves.
Much if not most major cave development and enlargement in the Black Hills region is thought to have taken place in the phreatic zone below the water table, with secondary enlargement and modification by vadose processes taking a much smaller role than in other typical karst areas.
Geologically speaking, much of the soluble bedrock in this uplift has until quite recently been covered by one or more hydrologically confining rock strata, and has most likely been under at least moderate thicknesses of cover during much of the speleogenetic process.
As a result, karst features may be evidenced only in the subsurface and the more typical expressions like sinkholes lapies and grikes may be totally absent in the surrounding terrain.
This is not to imply that the region does not have sinkholes and other observable surficial karst features, but only that they are far more limited in distribution than in most other major cave terrains, especially considering the number and size of the cave systems present.

Large phreatic passage Holloch Cave System, Switzerland


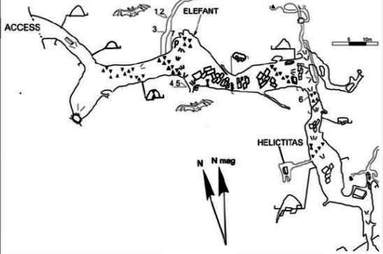
Keyhole Shaped Phreatic Tube with vadose "canyon" notch in base Elephant Cave, Wales

- Typical karstic features like sinkholes tend to be restricted to the Pahasapa limestone’s erosional margins nearest to its contact with the overlying Minnelusa formation. These features are associated with relatively recent supergene modification and enlargement of caves that owe their origins to dominantly phreatic processes.
- One of the most conspicuous absences from the Black Hills Karstic picture are the presence of well-developed sinkhole plains. This departure from the norm, however, is a direct consequence of the somewhat unique geological setting in which the most recently speleogenesis has been occurring.
- The cave systems exposed in the Black Hills uplift have not typically developed in a setting with a broad flat water table where fluctuations are primarily parallel to bedding planes as in Kentucky or Indiana. The current period of Karst development in the Black Hills has been within a moderately to locally steeply dipping confined aquifer on the flanks of an uplift with a substantial hydraulic gradient.
There has most certainly been more than one period of speleogenesis within the Madison formation, as there is observable stratigraphic evidence that the overlying Minnelusa formation has in some places infilled sinks and other karst features during its initial stages of sedimentation, and well before the deposition of the superjacent Permian and Jurassic age formations. This older Paleozoic age period of dissolution and cave development has often been referred to as “paleo-karst”.
At least some of the infilling of Paleozoic age karst, however, occurred well after the cycle of sedimentation was completed.
This period of infilling is associated with the concurrent dissolution of the gypsum and anhydrite in the Minnelusa during a much younger period of void formation that was simultaneously occurring within the underlying Madison (Pahasapa) Limestone. This period of karst development, which is responsible for most of the caves and karst found in the region today, is sometimes referred to as “neo-karst”.
- In the Black Hills region, karst features can most commonly be seen in the sides of drainages and on the surrounding bluffs. In some drainages, the sheer volume of observable solution enlarged fractures, voids, pockets, and dog-tooth spar incrustations is staggering. In other drainages these features are more subtle, but wherever a cross section of the upper part of the Pahasapa limestone is exposed they are omnipresent. Most all of these void features have phreatic characteristics and morphologies, and widespread dissolution within the upper 1/3 to 1/2 of the formation is indicated at one or more times since the formations deposition.
- The abundant pore space in the upper portion of the Madison in this area has also been found well out into the surrounding plains in deep drill holes; it is likely the result of older periods of speleogenesis as well as the ongoing process of de-dolomitization recognized throughout the aquifer’s 5 state area.

Exposure of the upper portion of the Pahasapa Limestone in Little Elk Creek canyon showing its' mega-porous character
- The widespread evidence of solutional activity within the Madison Group is what has made it the highly productive aquifer that it is. The extensive volume of interconnected pore space in this aquifer affords for rapid infiltration and recharge within the uplifted source areas of the Black Hills and Bighorn Mountains.
- Most (but arguably not all ) of the Paleozoic age cave systems (aka Paleokarst), appear to have been long ago filled with sediment and precipitated minerals. The bulk of the cave developments currently found in the region are probably post Laramide (< 65 My) in age, and were formed at some time after the Eocene (< 55 My) epoch.
Fold and Fault Related Systems
The fold and fault systems on the margins of the Black Hills have substantially enhanced the porosity and permeability along these discrete and highly localized structural trends. The structural deformation adjacent to faults and folds also provides for the abundant low angle bedding-plane fissures and high angle stress fractures needed to develop large scale cross and between channel flow paths. Within these zones of enhanced porosity and permeability, any actively forming and/or preexisting phreatic channels can undergo significant enlargement and develop interconnections into complex three dimensional networks with a substantially greater volume.
The complex geometric style of these caves suggests that passage development involved numerous interconnected phreatic loops in a confined aquifer setting. This environment implies one in which there was significant structural preparation, a substantial hydraulic gradient, high head pressure, variable but slow flow rates and a base level outlet to the system at significant distance from the source area of the solutions.
A substantial percentage of the passages in Black Hills caves tend to have low, flattened and anastomosing phreatic characteristics, and it is likely that the overlying confining layers were still largely in place during their formation. These passages are generally parallel or subparallel to bedding planes and do not typically “daylight” against the upper contact of the limestone in epigenetic sinks and/or other surface related features.
Many of the larger caves in the Black Hills uplift are labyrinthine style systems, with multiple levels and a highly complex series of interconnecting passages and loops. The passage geometries in these complex caves often mimic the trends of faults, folds and cross structures within the immediate area, suggesting a strong structural control on their development. A partial list of the complex multilevel caves currently explored in the Black Hills region includes such examples as:
Jewel Cave
Bethlehem Cave
Wind Cave
Brook’s Cave
Stagebarn Cave
Wildcate Cave
Reed’s Cave
Nameless Cave
The largest explored Black Hills caves tend to be spatially associated with major structural trends nearest to the flanks of the uplift. Many large (and small) cave geometries closely follow the trends of these major features which strongly suggests that a substantial period of their growth was post-deformational.
The diagram below illustrates an idealized monoclinal fold along the margins of the Black Hills Uplift. This situation represents a structural regime similar to that present during the speleogenesis of Stagebarn, Bethlehem and Wonderland caves. Although the example illustrated here is that of a monocline, similar types of structural regimes are also expected to be associated with any major fold, fault, dome or subsidence basin located marginal or internal to the Black Hills uplift.
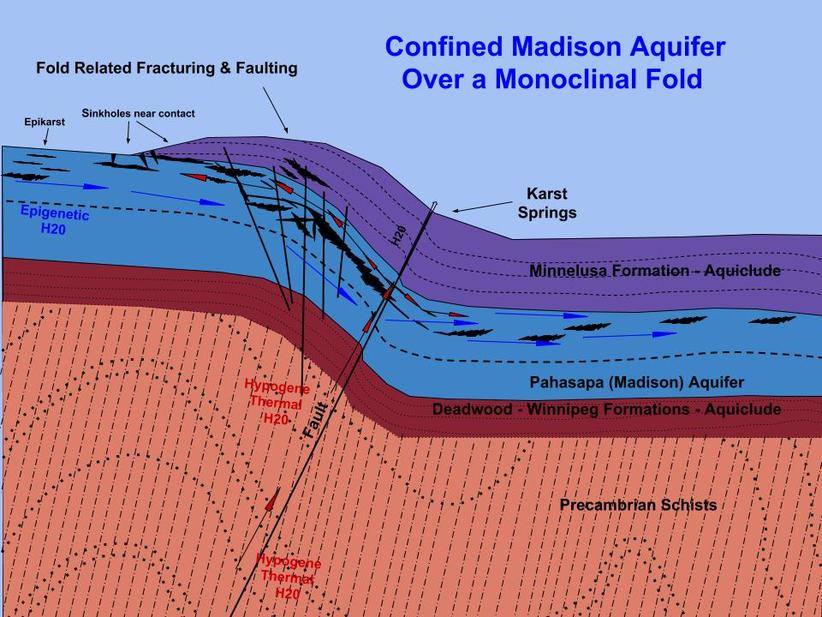
- If you look closely at this diagram, you will notice that both surface derived (epigene/supergene) and subsurface (hypogene) thermal waters are shown as potential sources for the speleogenetic solutions. These two end member represent the current thoughts on the nature of the driving force for the cave forming process.
- The temperature, geochemistry and source of the solutions involved in the most recent period of speleogenesis continue to be arguable (imagine that!), as are the depth below the water table/piezometric surface and/or the degree of confinement for the host aquifer. The structural control, on the other hand, is hard to refute in most every moderate to large sized cave in the region, regardless of the source and nature of the solutions involved.
The following two diagrams show caves that are spatially associated with major folds and illustrate that the expected tension and shear directions associated with these major structures parallels the geometry of a significant number of the phreatic passages in these caves. This correlation suggests that these caves are post deformational and that their speleogenesis can be related, at least in part, to the nearby structures.
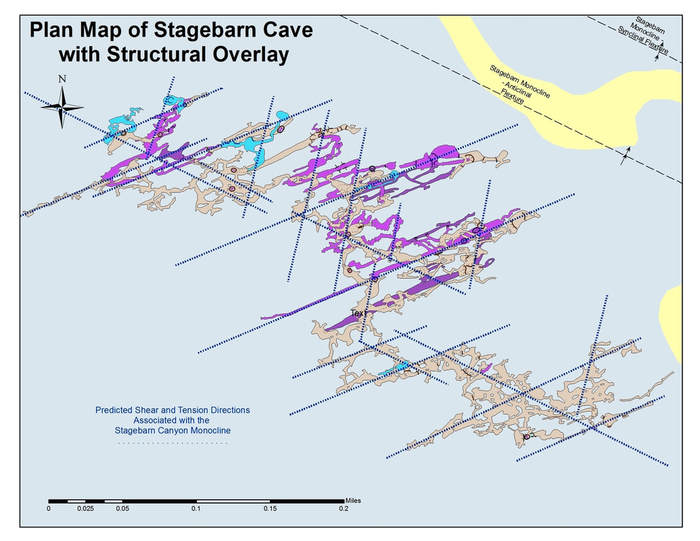
Stagebarn Caverns showing phreatic passage alignment with predicted tension and shears directions associated with the adjacent Stagebarn Canyon Monocline.

Bethlehem Cave showing phreatic passage alignment with the predicted tension and shears directions associated with the nearby Piedmont Monocline.
The anticlinal flexture of the monocline is directly south approximately 1500′. The synclinal flexture is unmapped, but likely trends through the cave area.
The Jewel Cave Structural System
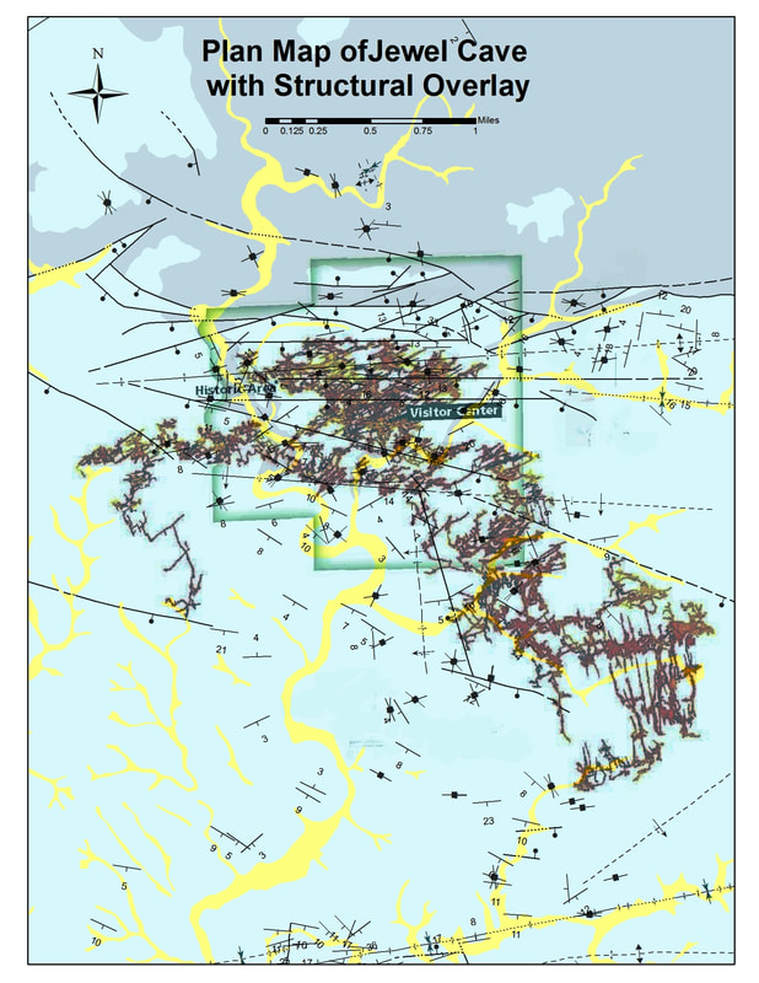
This overlay of the surface structural map of the jewel cave area with the mapped passages in the cave shows a very close correspondence between the fault and passage trends.
- North of the main fault system and its’ splays on the northern margins of the cave network, all the known caves are only a few hundred feet in length at best.
- South of the fault system there is a complex of over 194 miles (and growing) of interconnected passages.
- The majority of these passages have orientations that correspond closely to fault and fold orientations and their associated stress related fissures and fractures.
The Wind Cave Structural System
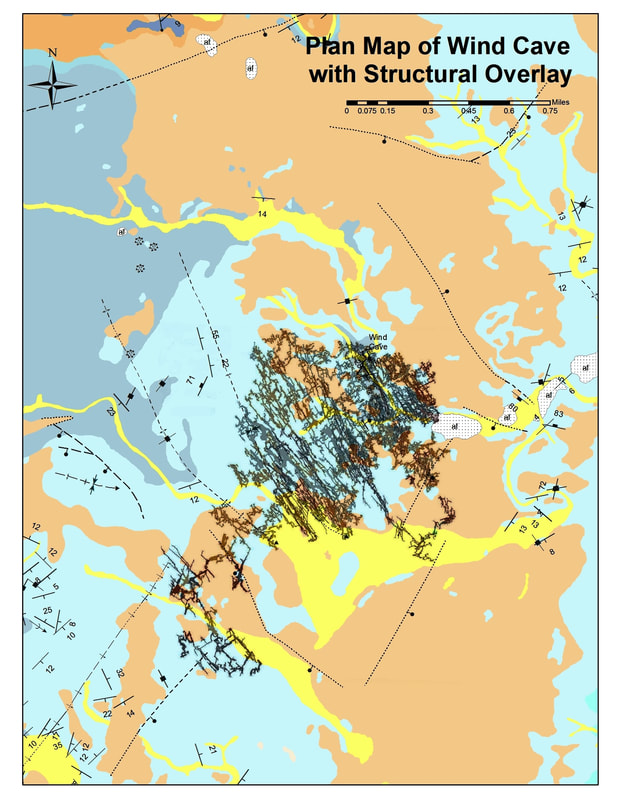
The Wind Cave system is spatially associated with a series of folds and faults associated with the Pringle Fault to the north. The cave is dominantly developed on the NE limb of an anticline. Surface structures are difficult to map in this area due to large percentage of unconsolidated sediments that cover the bedrock in this area. All of the major structures mapped on the surface or underground, however, are oriented in a manner consistent with their having been a strong control on the development of the cave’s phreatic network. (My opinion…there are others)
The following diagram shows a closeup of the Wind Cave passage network. All of the major NW trending passages/galleries parallel the Anticline and Syncline that trend through the cave system. Most all of the cross passages are similarly oriented to the southwest, which is parallel to the Pringle fault and a series of associated faults close to the southern end of the mapped network.
Source of the Solutions
Ascension (Hypogene) vs. Descension (Supergene)
With respect to groundwater, the genesis of Black Hills caves has been attributed to a number of different theories and combinations thereof. These include:
- Dissolution by typical surface derived (epigenetic or supergene) carbonic acid enriched precipitation, as is the origin of most other karst areas.
- Dissolution due to the recharge and fluctuations thereof near the uppermost edge of the confined artesian Madison aquifer.
- Dissolution due to mixing of surface related and formation waters within the Madison formation.
- Attack from “below” by hypogene (subsurface derived) waters ascending and/or migrating laterally into the confined Madison aquifer.
The available evidence (in my opinion) suggests that varying combinations of “hypogene” waters and supergene solutions are responsible for the most recent period of speleogenesis.
- Hypogene solutions (thermal of otherwise) refers to waters that are derived from other than the immediately overlying “recharge area” and that have ascended or moved laterally along structural and stratigraphic pathways through the aquifer and resulted in cave development. This usage of hypogene adheres to a strictly hydrogeological definition of the term, irrespective of any geochemical or thermal implications. As such, these waters may or may not be of a thermal nature, and can have either concentrated or dilute chemical compositions. The defining criteria is that the dissolution process does not involve waters derived from the immediately overlying recharge area.
- Surface derived supergene/epigene carbonic acid enriched solutions from soil zone and atmospheric sources have doubtless been responsible for Black Hills cave genesis in some areas, as well. The term epigene (or more properly, supergene) refers to those processes that originate at the surface and work their way downward. This mode of speleogenesis is the most generally accepted means of cave development for karst areas worldwide, but it is certainly not the only way that caves can form, nor is it the most prevalent mode in this region.
As to the thermal. non-thermal aspects of any of the ascending or laterally migrating solutions involved, the Black Hills currently has a relatively high geothermal gradient and probably has had since at least the Eocene epoch, so it is not unreasonable to expect the involvement of thermal waters at some level in episodes of speleogenesis since that time.
As for the geochemical nature of the lixiviants involved or the involvement of any “hydrothermal” solutions or concentrated formation waters, that question is open to speculation, but is certainly not unreasonable. There are many areas within the present day Madison aquifer with formation waters that qualify as brines and there is widespread evidence of hydrothermal solutions and their affects in the northern hills and Edgemont areas, so the possibility cannot be discounted.
I have a bit more observation and geochemical testing to do before I rant on further on that subject, but I rather lean towards the involvement of a thermal hypogene component due to its simplicity and a personal bias towards the validity and liberal application of Occam’s razor until proven otherwise.
Definitely more about this later… particularly if I can arrange for some fluid inclusion studies of dog-tooth spar from more than just one Black Hills cave…. like I said, this page is a work in progress.
Final (for now) Thoughts
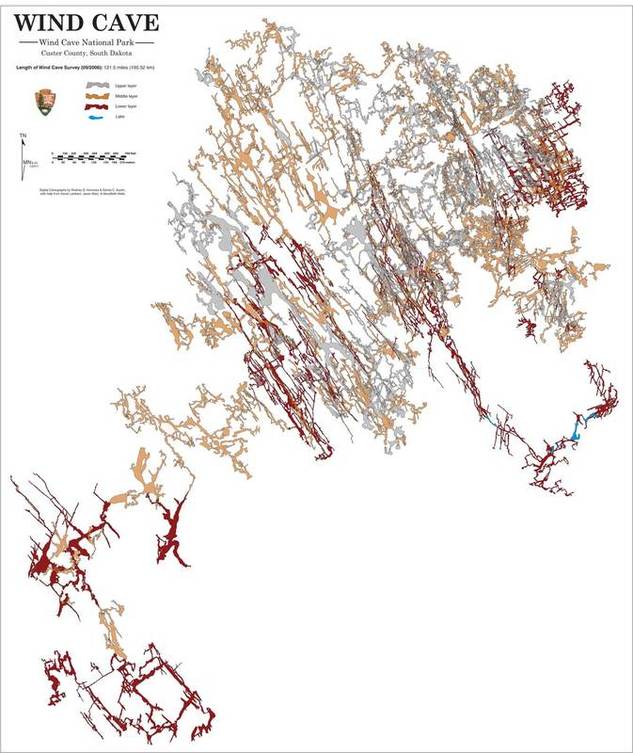
The Madison (Pahasapa) limestone contains over 400 known caves within the Black Hills area. At least two of these caves contain more than 100 miles of mapped passageways each and are currently ranked as the 2nd and 6th longest cave systems known on earth. Many others in the hills region are a mile or more in length, and the list is growing every year.
- The full extent of most of the hill’s larger caves systems are as yet unknown, and the potential for further exploration and discovery is without any doubt, second to none, both within these caves and elsewhere within the Hills.
In my opinion, we most likely have the largest cave system in the world under us… maybe even two or three of the top five. We just need to keep pushing those leads, walking those ridges, looking in the right places and using our heads and the best available science to guide our efforts.

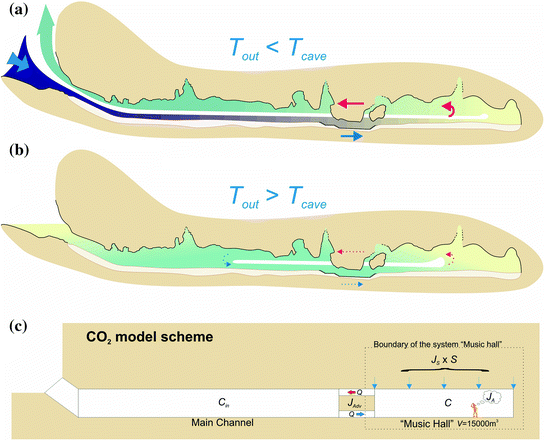
Void Formation
Karst and Caves features are all related to the chemical solution and dissolution processes that modify and sculpture all common soluble bedrock types. These susceptible rock types include rock salt (halite/sylvite), potash, trona, gypsum , dolomite and limestone.

In the case of the highly soluble evaporites like Halite, Potash and Trona, the formation of solution pockets, sinks and in some cases caves, is just a matter of water solubility. Ambient temperature precipitation and groundwater are highly undersaturated with respect to these halide and bicarbonate compounds and will rapidly dissolve large quantities of in-situ rock along fractures, bedding planes and surface exposures.Subsurface solution features within these rock types tend to be small, fragile and very short lived due to their high water solubility, but these rock types readily form karst solution pinnacles on surface exposures. These features, however, are generally only well developed and observable in extremely dry environments.
In the region immediately surrounding the Black Hills Uplift, there are no widespread occurrences of these types of evaporite deposits, but such evaporitic minerals do occur as minor admixtures in some of the beds in both the Spearfish and Minnelusa formations. Dissolution of these minerals along with the gypsum layers in which they occur has had a pronounced effect on the karst features found in these two formations.
Gypsum (CaSO4 · 2H2O) with lesser anhydrite (CaSO4) occurs within several formations exposed in and around the Black Hills area. These minerals are also water soluble, but far less so than the other salts mentioned above. These two sulfate compounds have less than 1% of the water solubility of the halide and bicarbonate salts. They are, however, considerably more soluble at higher temperatures and especially in the presence of acidic solutions. Rising thermal water have both of these attributes and can produce very significant karst within rocks of this type. They more commonly, however, can be seen to undergo dissolution in the presence of ambient temperature meteoric and ground waters, albeit much more slowly. The karst and caves that locally develop in gypsum tend to be much more extensive and longer lived than those in the other rock salts, because of the higher mechanical stability that comes with being less soluble. Even in view of this higher stability, however, gypsum caves are quite fragile and highly prone to breakdowns relative to limestone caves.

The Spearfish formation contains a thick bed of gypsum near it’s top and has stringers and thin layers of gypsum throughout its entire thickness. This formation rings the Black Hills uplift as the “Red Valley” or “Racetrack” as it is locally known. This unit contains widespread karst features that include solution domes, breccia pipes, sinkholes and local caves, particularly where the beds are intercepted or transected by flowing springs.
Most all of the speleologically significant karst in the Black Hills is contained within the Limestone strata of the Madison (Pahasapa) Limestone. Limestone is almost entirely composed of the calcium and magnesium carbonate minerals, calcite (CaCO3), aragonite (CaCO3) and dolomite (CaMg(CO3)2). All of these minerals are sparingly soluble to insoluble in water at surface and near subsurface temperatures. Limestones will, however, dissolve measurably in the presence of soil and atmospherically derived acids when adequate water is available, and this is the basis for the primary dissolution cycle of speleogenesis.
The Primary Dissolution Reaction
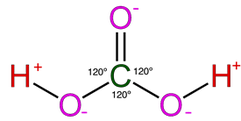
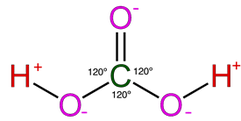
Most of the process of dissolution is accomplished through the action of carbonic acids (H2CO3) which form from carbon dioxide and water in the atmosphere and soil. Both carbon dioxide and water are highly polar molecules and dissociate into ions rapidly and are extremely stable under normal speleogenetic environmental conditions.
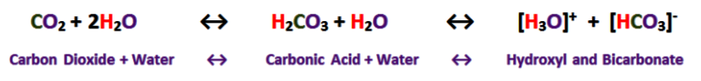
The following shows the carbonic acid – calcium carbonate dissolution reaction. This the most prevalent reaction taking place, but not the only acid mediated decomposition possible within the dissolution cycle, however.
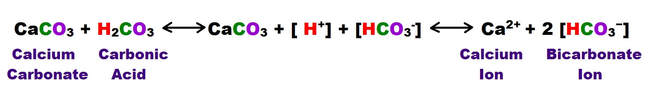
There can also be major contributions from the soluble organic acids that are produced by biologic activity within the soil. These acids consist largely of complex mixtures of polymeric compounds.
- Plant roots release acetic acid and citric acid
- Soil microbes produce fulvic acids, humic acids and phenolic acids (tannins)
- Fungi produce oxalic acid
These acidic compounds affect carbonate dissolution by increasing the total acidity of the soil. They also contribute to breakdown of inorganic minerals by acting as chelators, a class of compounds that surrounds and mobilizes metals in solution. The concentration of the soil acids in the epikarstic zone can sometimes be quite high, and they definitely assist in keeping the available hydroxyl ion concentration [H3O+] elevated within the epikarstic zone.
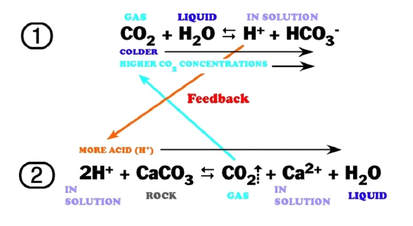
The following reaction shows how carbonic acid reacts with the calcium carbonate in limestone. Gases like CO2, N2 and O2 become increasingly soluble in water as the temperature drops or as the pressure increases. Both of these conditions are met as water sinks from the upper epikarstic zone into the cooler vadose and phreatic zones at depth. Furthermore, as calcium carbonate dissolves, it releases more CO2, and induces a feedback loop that generates more carbonic acid as long as there is adequate water available for the reaction to proceed.
Note that the hydroxyl Ion [H3O+] is shown in the above diagram in standard ionic shorthand notation as H+. From a chemical reaction standpoint, these forms are equivalent .
The effects of increased pressure on the solubility of carbon dioxide as the solution works its way down the solution column can be quite significant. Within the first 30 feet of a saturated column of water at 20°C, carbon dioxide solubility increases by a factor of 20 and a liter of water can readily dissolve as much as 0.7 liters of gas at the bottom of the column.
The effect of this increased solubility means that as more carbonate is dissolved, more CO2 is released into the solution and it stays dissolved,where it forms more carbonic acid and increases the capacity of the solution to dissolve additional carbonate. Although this process takes place throughout the entire flow path, the cycling and recycling of the CO2 takes place most efficiently in the phreatic zone below the water table.
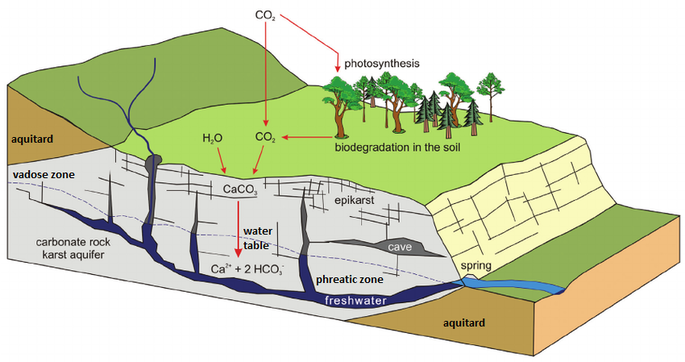
The above diagram shows a general schematic of the carbon dioxide – bicarbonate cycle within the epikarstic, vadose and phreatic zones.
Secondary Dissolution Reactions
There are countless other reactions that take place within the karstic cycle, a number of which can enhance the dissolution process within the carbonate bedrock. Other reactions can either slow down the process or change the dissolving solutions in ways that have effects in the down-gradient portion of the system. The following sections outline a few of the characteristics of the major reaction types, and the effects they typically have on the overall process.
Hydrolysis Reactions
Many of these reactions involve the reactions of carbonic and other acids with the inorganic silicate and oxide minerals grains that are typically dispersed in varying proportions throughout the limestone matrix. This process involves acidic hydroxyl ions and releases small quantities of metal ions into solution in a process known as hydrolysis. These reactions are acid consumers and initially tend to slow the overall dissolution process by limiting the availability of acidic hydroxyl ions. The following reactions illustrate two common hydrolysis reactions that produce one of the clays commonly found in karstic soils.


Because these reactions produce hydroscopic clay minerals, they help in the long run to increase the retention time of water where they are present. This decrease in flow rate often aids in the further decomposition of carbonates by slowing the rate of acidic hydroxyl [H3O+] ions through the system and allowing more time for reactions to take place.
Additional hydrolysis reactions occur that involve the metal ions formed in the types of reactions described above. These ionic exchanges in solution cause the formation of hydroxide (OH-) bearing precipitates and regenerate the acidity [H+] of the solution. This process most commonly occurs where the mixing of descending acidic waters blend with less acidic groundwater, at or below the water table. The result is the precipitation of metal hydroxides that are incorporated into mineral growths, crystal structures and surface coatings. A few common examples of these hydrolysis reactions are shown in the following list.
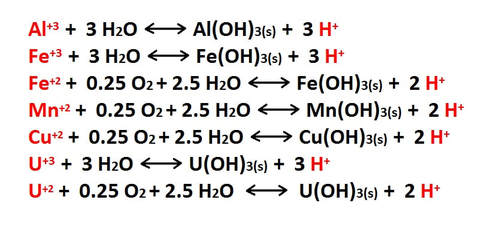
Many metal hydroxides are quite colorful, and the ions that produce them are often referred to as “chromophores“. These ions are important factors in the development of the colorful formations found in caves worldwide.
Metal ions available for hydrolysis are not only derived from the inorganic minerals and oxides found in carbonate sediments, but also from the structure of the carbonate minerals themselves. Any metallic ion with a +2 charge can substitute for the calcium in carbonate minerals, and impurities of this type are found at various levels in most limestones and dolomites. The list above is my no means all inclusive.
Both copper (Cu+2) and uranium (U+2) ions are typically present in only trace quantities in carbonates, but they can be important components in the precipitation of late stage minerals. Uranium in particular is a widespread trace constituent in limestones and has a major role in determining the geochemical makeup of cave atmospheres.
Oxidation Reactions
There are also reactions involving dissolved oxygen that attack any sulfide mineral growths that may be present within the limestone. Diagenetic sulfide grains are quite common within carbonate sediments and related to their compaction and aging process. Many carbonates have reduced carbon that is trapped in their sediments during sedimentation. These sulfides can also be related to the reducing effects of post-depositional petrochemical migration through the host rock’s pore space. In some cases, particularly in the vicinity of hot springs or igneous intrusions, there may also be hydrothermal sulfide grains that were introduced much later as disseminations and replacements within the fractures and bedding planes of the host carbonate rock.
All oxidation reactions involving sulfides have the ability to release strong acids into solution. These acids will profoundly increase the rate of carbonate dissolution wherever they are present. These reactions also release alkali, alkaline-earth and transition metals into solution which are incorporated into crystal structures in minerals that precipitate further down through the system (See “Hydrolysis Reactions” above and “The Precipitation Cycle” link on the bottom of this page). A few of the more typical sulfide oxidation reactions are shown in the figure below.
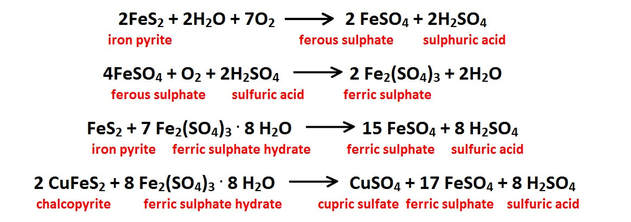
All of the reactions shown either produce or consume sulfuric acid (H2SO4); the net effect is that much more of this strong acid is produced in this reaction chain than is used up. The acidic hydroxyl ions [H3O+] released from these oxidation reactions lower the solution pH, and increases the fluids’ ability to dissolve additional carbonate bedrock on it’s downward path to the water table and on into the phreatic zone below.
Speleogens are the name given to the class of morphological feature that formed as a direct result of the dissolutional process. They are a prominent feature in Black Hills caves, and are are a particularly common feature within the phreatic portions of cave systems in general.
Speleogens are residual relief features on the surfaces of passage walls left over from the dissolutional process. These features can also be due to corrosion by flowing water and or unusual forms of chemical activity such as sulfide dissolution.
They consist of readily recognizable shapes that include such features as scallops, fluted surfaces, anastomoses, meander niches, solutional siphons, petromorphs and rock pendants.
The vast majority of precipitated growths and minerals (speleothems) are calcareous in nature, and composed of either calcium carbonate in the form of calcite or aragonite, or calcium sulfate in the form of gypsum. All of these speleothems form from the products of the primary and secondary carbonate dissolution reactions as were outlined in the previous section. The primary reaction related to carbonic acid dissolution is restated below:
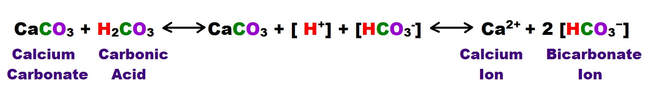
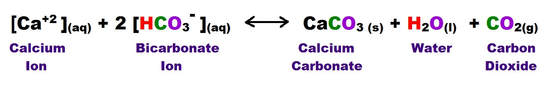
Degassing of the downward moving groundwater is one of the principal triggers for calcite precipitation. The carbon dioxide content of the groundwater that seeps into an open space can be as much as 250 times higher than that of the cavity. When a gas charged solution reaches an open area, the decrease in confining pressure allows the dissolved CO2 to rapidly escape. This process results in the precipitation of CaCO3, which is most typically in the form of the mineral calcite.

Over time the accumulation of these calcitic precipitates will form dripstone, stalagmites, stalactites and all the other commonly observed carbonate bearing speleothems within available open space.
As the partially degassed water continues on towards the water table and beyond, it does not initially carry as much calcite in solution. As a result of degassing, however, the CO2 content of the atmosphere in the cavity rises, which will readily diffuse into any undersaturated aqueous solutions therein and spur additional dissolution. This is particularly true when rock and aqueous solutions within the cavity are at lower temperatures than the incoming solutions.
The second major method for calcite deposition is from evaporation of the carbonate saturated groundwater when it encounters open air space.
Either way, calcitic formations are deposited that are referred to as speleothems. This term specifically refers to those features formed later than the cavity in which they are found.
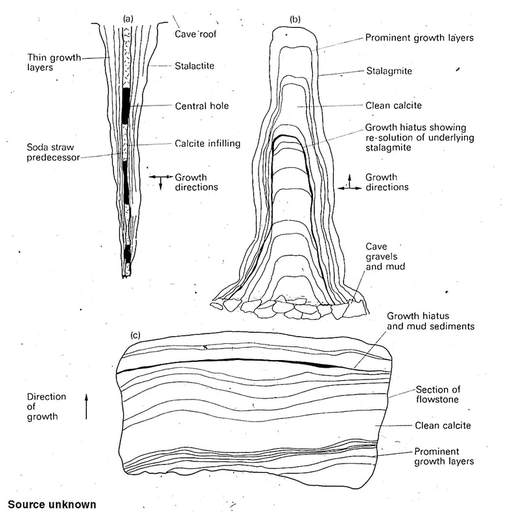
Although many of the more familiar precipitation features are vadose in origin, major phases of calcite growth occur at or below the water table in the phreatic zone in many systems. In the Black Hills, dog-tooth calcitic spar precipitated at or below the water table is far and away the most abundant mineral produced.
Caves each have their own unique atmospheric chemistry and a distinct barometric response to external pressure changes. These characteristic footprints can vary from simplistic linear responses to highly complex multivariate systems with numerous feedback loops. The following sections discuss first the causes of airflow within caves, and then the nature of that air from a geochemical standpoint.
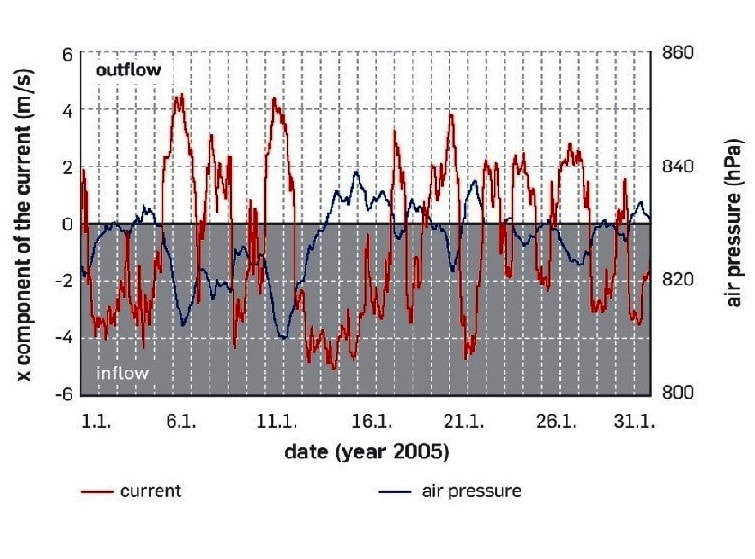
Jewel Cave Barometric Data - Andreas Pflitsch et al.
The last section will outline what is currently known about these atmospheric factors in Black Hills caves, who is doing the research, and a bit about the ongoing projects here in the Hills.
Cave Barometry & Airflow
In general, the larger the cave’s volume, the more substantial will be the flow of air in and out with a given change in barometric pressure. Other factors that determine flow rate are the size, number and elevations of any openings back to the surface.
The response observed from barometric and other physical changes depends on a number of complexly interdependent factors that can either accentuate or retard flow under a given set of conditions. A cave’s size, geometry, wall roughness and passage tortuosity all effect airflow characteristics, as do the relative sizes and proximity of openings to one another.
Caves can breathe for a number of different reasons. Barometric shifts are one of the primary drivers for flow, but movement may also be induced from chimney effects and wind variations when there is an elevation difference between multiple openings.
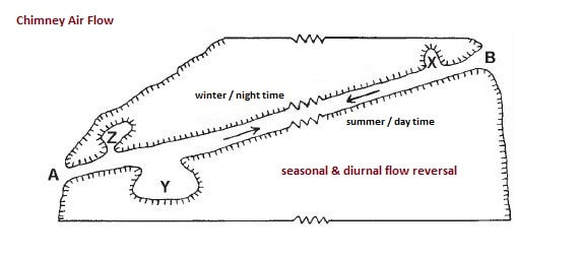
A difference in air temperature and/or density between openings or even portions of an opening can lead to observable airflow.
These air density differences are usually small and tend to be seasonal and/or diurnal in nature.
Low to moderate levels of air flow can occur in response to daily and seasonal temperature fluctuations, depending on cave size and geometry.
Cold air can be trapped in low spots and lead to ice accumulations where a single entrance is present and the interior recesses are significantly below the opening.
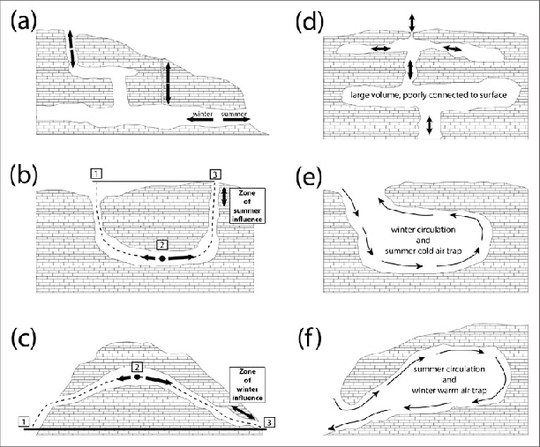
(a) Chimney Flow
(b) Restricted Gravity Well / Sink
(c) Barometric Siphon and Chimney Flow
(d) Blow Hole
(e) Unrestricted Gravity Well / Sink
(f) Barometric bell
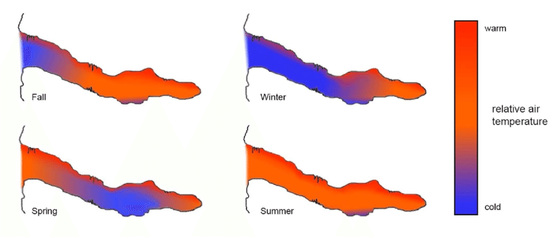
The adjacent diagram shows the thermal regime in a small pocket type cave. Air movement in response to seasonal thermal patterns will cause breathing in during the fall and winter months and out during the spring and fall seasons.
It is not uncommon for a breathing cave to have contributions to it’s flow patterns from more than one of the above mechanisms. Careful observations and flow measurements are usually required to separate the effects of each.
In a barometric cave where the number of openings are few, and in which the effects of chimney flow are minimal to absent, the inflow and outflow rates will be directly related to the system’s volume. This means that the cave’s ultimate volume or “size” can be estimated for the portions of the system above the water table.
Additionally, if enough is known about the average passage characteristics in the portions that are mapped, a tentative”length” can be approximated for the system. Cave barometry is an extremely powerful tool in the assessment of the significance and size of cave resources.
Cave Atmospheric Chemistry
The chemistry of spelean atmospheres are by no means static or simple in terms of gaseous makeup. Depending on the types of carbonates present, the minor and trace element contents of those carbonates and their proximity to other nearby lithologies, a cave’s geochemical signature can vary from relatively uniform to highly complex.
In general, larger caves with fewer entrances have more complex and unique atmospheres, but smaller caves can have distinct and surprisingly multifaceted signatures as well.
All of the gases present in the outside atmosphere are also found in the average cave system. Given this framework, there will typically be compositional shifts within the cave’s atmosphere that have been induced by the geochemistry and biochemistry of the wall rock and biota present. The cave’s barometry, geometry and temperature also play a key role in determining the flow and exchange rates of the interior air with the outside as well as the diffusion of cave specific gaseous components outwards.
The following chart shows all of the major atmospheric gases and a suite of others that have been recognized and studied in various cave systems worldwide. This list is by no means all inclusive, as many others are possible. The average atmospheric value for each gas is listed as an index for assessing possible anomalous concentrations in a cave’s environment. Some of the most likely sources for an anomalous gas concentration are also given.
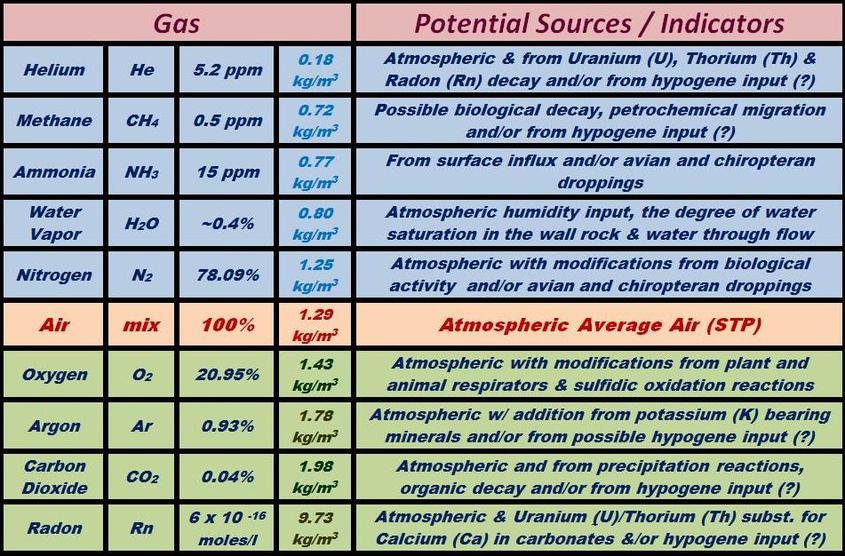
The lighter-than-air gases are all highlighted in blue at the top of the list, and will diffuse rapidly if generated within the cave environment. Anomalous levels of these gases in a cave’s atmosphere would therefore indicate a relatively high inward flux rate, or a very slow rate of exchange with the surface. The light gases will typically have the most variable concentrations within cave air over time due to their lower densities and higher diffusion rates relative to the average air mixture.
The heavier-than-air gases are all highlighted in green at the bottom of the chart, and in general will tend to be enriched in cave environments that have passages below the levels of any entrances. Heavy gases usually have the highest residence time and consistent gas compositions within a given cave, as the rates of exchange and diffusion are slower and they often have higher concentrations in areas of stagnated flow. The three heavier than air gases that have the highest probability of enrichment in spelean environments are:
-
Radon in Cave Atmospheres
Radon Chemistry and Radiochemistry
Radon (Rn) is the heaviest of the naturally occurring noble or inert gases. This radioactive gaseous element has no stable form in nature but is rather a mixture of its 36 naturally occurring albeit short-lived radioactive isotopes. The longest lasting of these isotopes (Rn 222) has a ½ life of just 3.8 days, so at any given time there is not much to be found in nature and in fact, there are only a few tens of grams of Radon present at any time in all of the Earth’s atmosphere.
This does not seem like much of an abundance to be concerned with, but this merely represents the steady-state flux of a gas that is constantly being regenerated. As such, it still represents an important and omnipresent atmospheric component. Radon reaches a steady-state concentration quickly and does not build up past a certain level due to this constant decay, so it is always present and generally found to be at a very constant and uniform concentration within the atmosphere as a whole.
Within caves, however, there is far more variability than is seen within the atmosphere, and that variability can give valuable clues as to the atmospheric dynamics and passage geometries found within the cave system. Radon can also provide insights into the nature of the solutions and bedrock geochemistry involved in groundwater evolution and the system’s speleogenesis. Radon is moderately soluble in groundwater at normal spelean environmental temperatures and pressures. As a consequence, its concentration in subsurface atmospheres varies with the degree of ventilation, groundwater solution equilibrium, background Rn gas levels in the surrounding formations and temperature.
Like carbon dioxide, radon (Rn) concentrations in cave-air typically exhibit seasonal variations, with summer maxima and winter minima where there is at least some groundwater transport through the vadose horizon. Less variability is typical in systems that are completely dry, although ventilation, Rn gas values in the host rock and temperature related variation may still be evident.
The relatively steady concentration of ambient radon gas in both above ground and subsurface atmospheres is due to constant additions from the radioactive decay of uranium (U) and thorium (Th) found in natural materials. The decay chains for these two actinide elements are a significant source of the normal background radiation in our environment, and the origin of most of the helium (He) present within our atmosphere.
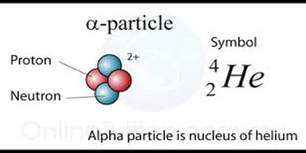
One of the principal ways in which radioactive elements decay is by alpha particle emission. An alpha particle is nothing more nor less than a Helium (He) ion that quickly captures a couple of stray electrons to become helium (He) gas.
Although Radon is radioactive, it is still a nonreactive noble gas from a chemical standpoint, which means that it is inert and essentially non-toxic from purely chemical considerations. It does not dissolve readily in water, nor does it react chemically with organic tissues, or is it absorbed or used in any way by the body. Radon has absolutely no tendency to accumulate in the environment or within living organisms, either. Regardless of its seemingly innocuous chemical behavior, however, radon’s radiochemical activity must always be addressed when its effects on living organisms are being considered.
The adjacent nuclear reaction is the portion of the decay of Uranium 238 into Lead 206 that produces Radon 222 gas.
Rn 222 is the most abundant of the radon isotopes and the only isotope that the name “radon” formally applies to, although in practice they are all usually lumped together under this name.Both uranium (U) and thorium (Th) readily substitute for the calcium (Ca) atoms found within mineral structures, and even though these two elements are found at very low levels in most rocks, enough radon is produced thereby to keep the background atmospheric concentration at about 6 x 10-16 moles/liter.
The equivalent to this chemical concentration is usually expressed as a radiochemical dosage when radon measurements are reported, however.
Here in the U.S., this value equates to 0.4 pCi /L (picocuries/liter); elsewhere in the world, it is expressed as 0.0148 Bq/m3 (Becquerels per cubic meter).
For those interested in the numbers, a picocurie is just 0.000,000,000,001 (one-trillionth) of a Curie, which is one of several internationally used units of radioactivity measurement.
A concentration of 1.0 pCi/L means that in one liter of air at that at level there will be about 2.2 radioactive disintegrations per minute.
At a level of 4.0 pCi/l, therefore, approximately 9-10 radioactive disintegrations will occur each minute per liter of air (see radon reference level below).
This amounts to about 1 per second within the average human lung volume of 6 liters.
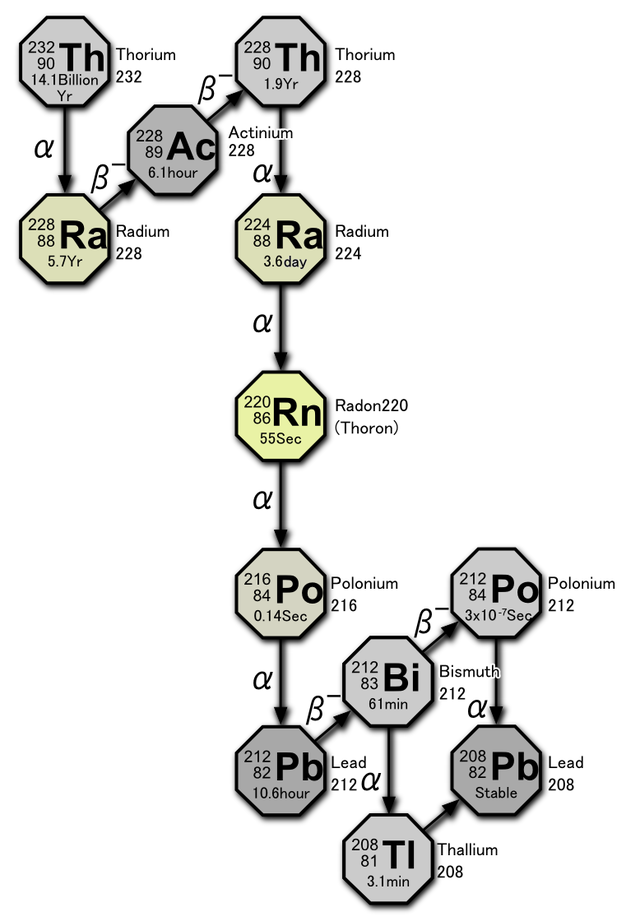
The adjacent nuclear reaction shows the portion of the decay of Thorium 232 into Lead 208 that produces Radon 220 gas.
Rn 220 is often referred to as “thoron” but due to its relatively low abundance (5-15%), it is typically lumped together with Rn 222 as “radon”.A quick comparison will show that there are approximately 27 Bq/m3 for each 1.0 pCi / L, so conversion from one unit to the other is quite straightforward.
Radon Reference Level
A reference value of 4.0 pCi/L – (108 Bq/m3) has been established by the Environmental Protection Agency (EPA) as the level at which radon levels are considered to exceed the “normal” or “acceptable” background range for living spaces; this concentration is about 10 times what background atmospheric levels are known to be. The reference value, however, is not based on any observational criteria or known set of physiological effects at that level. It is rather a concentration at which “concern” should be considered prudent, most likely because it represents an environment in which radon levels are a full order of magnitude (10X) higher than average background levels. Allowable workplace levels have been similarly set by the EPA at the higher value of ~15 pCi/L – (400 Bq/m3) which is about 4 times the level established for living spaces.
The health effects due to radon inhalation are typically estimated using the so-called “Linear No threshold” (LNT) relationship. This assumes that the radiation effects produced from low level irradiation can be predicted quantitatively by linear extrapolation of the effects produced with high level irradiation. This assumption implies, therefore, that any amount of radiation, however small, is potentially harmful.
The LNT hypothesis provided a decidedly conservative approach to radon risk assessment for the purpose of standard setting. However, its application has likely lead to some rather overly conservative radiation protection standards being put in place, particularly with respect to the dangers of low level irradiation. The LNT approach in this application has been repeatedly criticized due to the lack of sufficient definitive evidence required to support its use to assess the health effects of low-level radiation. The risks of lung cancer attributed to low level radon exposure are simple extrapolations from extremely high level (lethal) dosages, and the assumption of simple linearity is neither justified nor prudent based on the available evidence. https://pdfs.semanticscholar.org/404b/3d6f916752ff78864f16158e29db26454c20.pdf
The adjacent nuclear reaction shows the portion of the decay of Thorium 232 into Lead 208 that produces Radon 220 gas.
Rn 220 is often referred to as “thoron” but due to its relatively low abundance (5-15%), it is typically lumped together with Rn 222 as “radon”.There is quite a bit of variation in concentration seen at ground level in some areas which is primarily due to differences in local rock types and their geochemical affinities. With the measurements I have performed, the atmospheric average is more closely approached at a few meters above the ground.
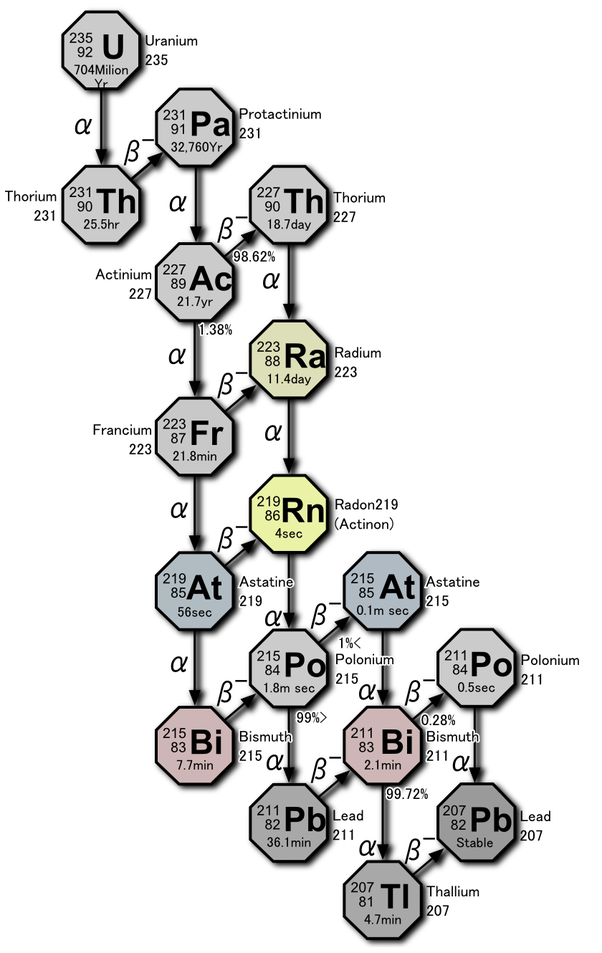
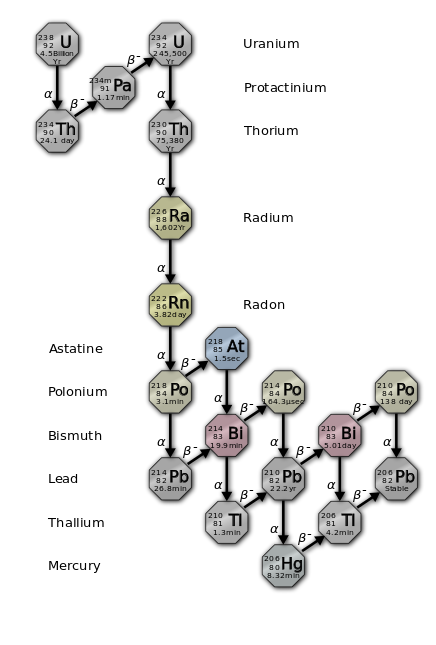
The following diagrams show the complex radiochemical mechanisms for uranium (U) and thorium (Th) decay, which transition through no fewer than 10 daughter forms each before they decay into one of several stable isotopes of lead (Pb). These decay chains each produce a single radon (Rn) daughter in the central part of the string, which makes the various radon isotopes the parents for all the reactions down-chain from that point.
The radon isotope phase is the only point within each chain where the product is a mobile gas, and the principal stage at which environmental dispersion takes place. All of the various daughter products are much less mobile post-transition metals that range from moderately to extremely toxic, both chemically and radiochemically. This is why radon exposure levels should always be considered when any long term extended exposures are a possibility.
Click on any of the above decay chain diagrams for more details
Radon gas is almost 8 times as dense as air and diffuses slowly, so it tends to become enriched in low spots and within closed spaces. Some would argue that this is not the case or even possible, but from a purely empirical standpoint, the low and closed spaces measured within caves follow this trend more often than not.
Radon exposure levels can become very significant when in a highly enriched closed space like a uranium mine, thorium mine, or within a well or basement dug into rocks enriched in the suite of lithophile elements that U and Th are typically associated with. Radiological exposures to radon are usually considered as averaged over time and can approach prohibitive levels with prolonged exposures at high concentration levels.
Radon in North American Caves
In general, radon concentrations in caves are higher than within open air settings, which is understandable considering that caves typically have restricted air exchange potential with the external atmosphere.
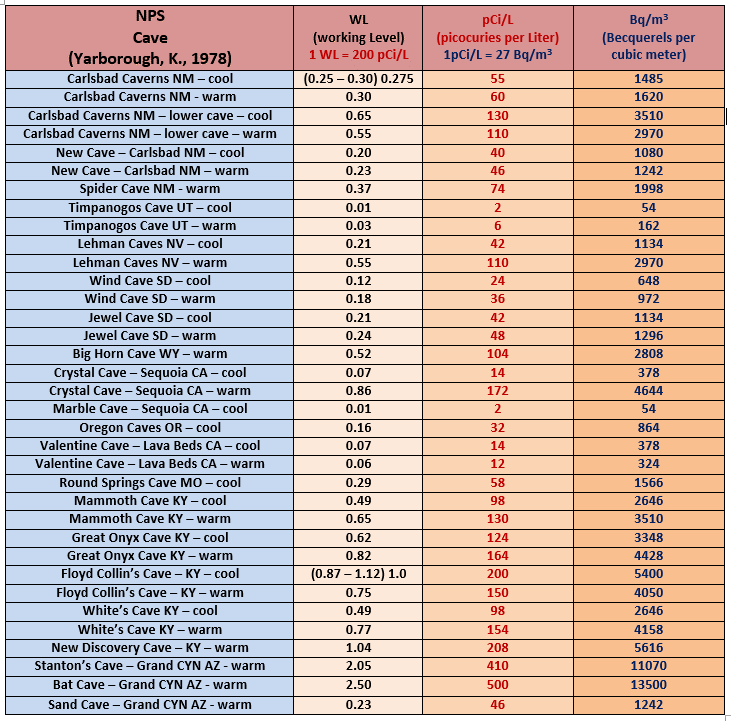
Radon is known to reach highly elevated concentrations in some North American caves (rarely up to 500 pCi/L and above), but in general American caves have somewhat lower Rn levels than have been reported in European caves and elsewhere.
Limestone has a higher background level of uranium than other sediments due to its high calcium (Ca) content, and therefor most limestone caves have radon levels that exceed atmospheric levels.
The primary determinant for finding elevated background levels, however, is the nature of the underlying and adjacent bedrock, as well as the geochemistry of the local groundwater. This is true within both cave-blessed and cave-deprived areas. To keep things in perspective, however, it best to be aware that many basements and the lower levels in well insulated homes will contain radon levels that are comparable with those found in many cave systems.
The only way to know for sure whether a particular cave has a high radon level is through testing. This is usually done through measuring long term averages at one or more locations within a cave or by spot checks in various locations at different times of the year.
The National Park Service (NPS) has done numerous long term radon monitoring studies in several of the nation’s largest cave systems which has looked at cumulative radon dosages over time for both its employees and visitors.
The EPA has done a similar analysis that also included recreational cavers. These studies concluded that neither employees nor frequent visitors or recreational cavers experienced significant increases in lifetime exposure levels, considering the short duration of most of their visits.
Radon in NPS Caves
Looking at the NPS sites evaluated in the chart above, there is some tendency for eastern and southwestern caves to have higher Rn levels than those in the west, but the data set is certainly not all inclusive. Some caves have quite a bit of variability in radon distribution, but others like Wind Cave have relatively homogeneous values.
Radon in North American Caves – Synopsis
Those of us that work in and or are frequent visitors to caves, therefore, are generally only rarely at a higher risk from these short term ventures into the Stygian depths. The possibility for encountering high Rn levels within a cave that has not been previously evaluated can usually be anticipated, however, based on local geology and nearby rock associations.
Radon tracking is not an imperative in most situations, however, due to the short term exposures that are generally experienced when visiting most caves. It is, however, advisable to consider when it is planned to visit a particular cave over an extended period of time.
Radon as a Cave Atmosphere and Geochemical Tracers
Aside form its obvious value for addressing health risks, radon testing and distribution profiling can also be an excellent tool for better understanding the dynamics of a complex cave system’s atmosphere, due to the element’s very high density and relatively slow diffusion characteristics. The complex patterns of flow, air exchange and stagnation can be in many cases better understood with information on the heavy gas components within a given system. To this end, radon is a a relatively easy, economical gas to measure and quantify. This is typically done through the use of short and long short term track etch monitors, as well by the use of hand-held continuously recording meters. Top of the line monitoring equipment can be quite pricey and is usually quite bulky, but a well protected hand-held device in a pelican case that is allowed to breath during spot checks works very well, gets the basic information desired and is a heck of lot more affordable.
Alpha Track Etch Type Radon Monitor
Handheld Radon Monitor
Thermal waters often have distinctive solute, gas and radioisotopic characteristics that may impart a distinctive fingerprint to the system’s groundwater and atmosphere. Radon analyses can in some cases help to determine the presence of hypogene components in the phreatic portions of a system where thermal waters may have had a hand in the dissolution process. This is particularly true if isotopic analysis are available, as groundwater radiochemistry can be be very distinctive depending on its source.
Hypogene Cave Features
Monitoring/profiling of radon levels has also provided insight into how elevated Rn levels within a cave may affect the distribution of its cavernicole (cave dwelling) inhabitants. Bats, in at least one study of four caves in Mexico, seem to have figured out that the highest radon areas in these caves are not the best areas for roosting and colonies. It would be very interesting to see whether this adaptability pattern extends to cave systems across North America and elsewhere.
Bat adaptability to High Radon in several Mexican Caves
Bats have relatively long lives (~20 years) and extremely high metabolisms, so all things being equal, one would expect high rates of radon-related mortality in these creatures due to the amount of time they spend in high radon environments. To the best of my available knowledge, this theory has not been thoroughly tested as of yet…hmm…sounds like a great PHD or post-doc project to me. So many questions…so little time!!
-
Carbon Dioxide in Cave Atmospheres
Carbon dioxide is by far the most important and fundamental of all the gases found in cave atmospheres. It is responsible for both the original formation of the cave’s open space and for the precipitation of the mineral formations that will in time refill them. Due to its genetic ties to the cave forming process, relatively high abundance in cave air and its rapid response to equilibrium shifts, carbon dioxide is an excellent tracer for the study of cave atmospheric dynamics.
Cave-air CO2 concentrations are almost always considerably higher than the current 390 parts per million (ppm) atmospheric average. Cave-air CO2 levels are often observed to rise with increased distance from surface openings, as well.
It is not uncommon for ambient CO2 levels to rise to several thousand ppm and more in some caves and several percent CO2, though rare, can occasionally be encountered in narrow enclosed spaces.
At concentrations up to 2%, very few effects other than a decrease in working capacity are experienced. When CO2 levels rise above 3%, however, highly labored breathing and skin tone changes are the warning signs of approaching blackouts and possible asphyxia.
Fortunately, extremely high CO2 levels are only very rarely encountered in caves, but it is good to be mindful of the possibility thereof, none-the-less. CO2 monitoring, if for no other reason than the recognition and avoidance of these rare situations, should be a consideration when entering newly discovered cave systems for the first time.
Cave-air CO2 levels, at any concentration level, are directly related to the speleogenetic process and are strongly affected by both internal and external atmospheric dynamics. Cave atmospheric CO2 levels typically vary with temperature, the degree of surface air advection into the system, local groundwater chemistry and the CO2 gas content of the surrounding bedrock.
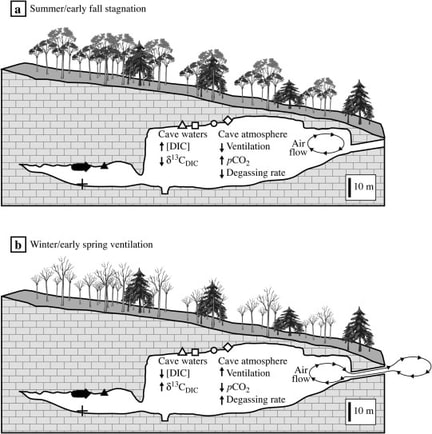
Due to the combined influences of these factors, the carbon dioxide levels within a cave will typically follow a definite pattern of seasonal and diurnal fluctuation, with significantly higher inner atmospheric levels during the warmer times of the day and year and lower levels during the cooler periods.
Seasonal Cave Air Variation
Dissolution mechanisms predominate during periods of high CO2 gas levels and precipitation typically takes place during periods of lower concentration, so these processes tend to follow the seasonal and diurnal patterns of CO2 fluctuation (see the section on reaction progress below).
Gases in general have much higher solubilities in cold water than in warm water, and the advected warmer air during the summer months also helps to seasonally increase the CO2 and all the other trace gas contents of cave-air.
The degree to which the gases present in the connected pore spaces of the surrounding formations controls gas levels within a cave are generally not well understood but are none-the-less likely to be significant.
Formational CO2 and other gases typically migrate along faults, bedding planes and other discontinuities within bedrock, just as groundwater or any other fluid will, and these transported gases may (often?) constitute a major component in cave-air chemistry.
Carbon Dioxide
Reaction Progress and CO2 Levels
The following two reaction series show the mechanisms responsible for cave dissolution and cave mineral precipitation, so they may be considered in light of how changing CO2 levels can affect the reaction progress in each cycle.
In the world of chemistry, Le Chatelier’s principle dictates that if you increase one of the reactants on the left side of a reversible reaction at equilibrium , it will drive the balance towards the products on the right. On the other hand, if you increase the amount of one of the products instead, it will shift the reaction back towards the left.
Carbon Dioxide
As a consequence, having a high and/or increasing CO2 level within a cave will tend to drive the reaction toward the right (product side) which favors the dissolution cycle, as outlined in the two linked reactions shown below.
The Dissolution Cycle
These same conditions will also simultaneously inhibit the precipitation cycle. This is because CO2 is one of the products on the right hand side of that reaction, so an increase in the CO2 level shifts the system back in the opposite direction towards the dissolved bicarbonate ion. This is illustrated in the precipitation reaction below.
As a consequence, having a high and/or increasing CO2 level within a cave will tend to drive the reaction toward the right (product side) which favors the dissolution cycle, as outlined in the two linked reactions shown below.
The Precipitation Cycle
Le Chatelier’s principle similarly dictates that decreasing the amount of one of the products on the right hand side of a reversible reaction at equilibrium will have the same effect as increasing one of the reactants and shifts the reaction towards the products.
Having a lower and/or decreasing CO2 level in a cave, therefore, will tend to favor the precipitation cycle and the growth of calcite and speleothems.
Although the speleogenetic processes of dissolution and precipitation take place over a wide range of environmental conditions, there generally appears to be an upper limit of about 4000 ppm CO2 in cave air for calcite precipitation to take place. Cave systems with higher year around average concentrations than this threshold value tend to have speleothems that no longer grow, or to be devoid of speleothems entirely.
Bethlehem Cave - Photo by Mike Hanson
-
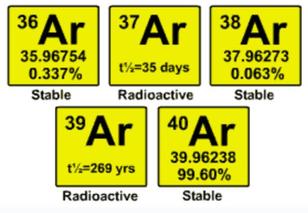
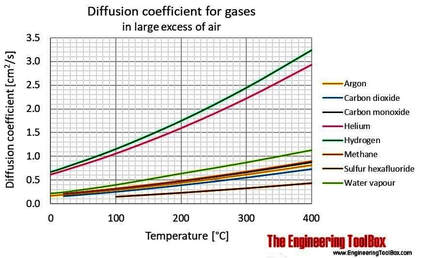
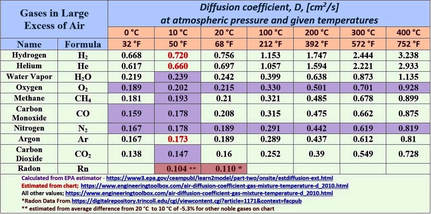
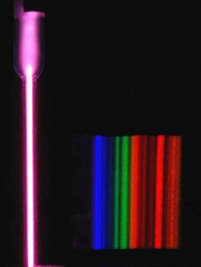
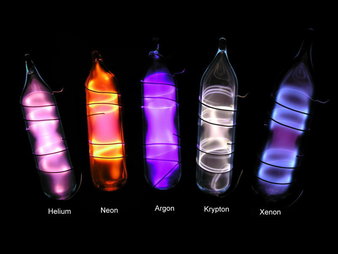
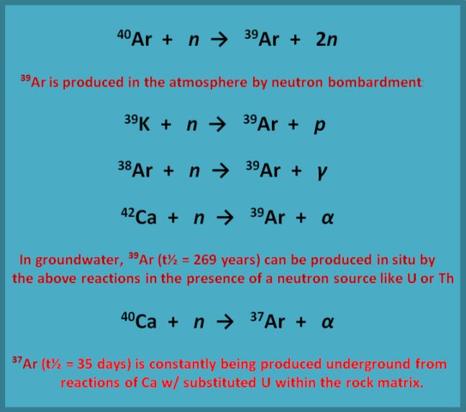
Argon in Cave Atmospheres
Argon (Ar) makes up 1.3% of our atmosphere by weight and 0.934 % by volume. It can also be found everywhere dispersed through the pore space of rocks within the earth’s interior. Although held tightly within defects and holes within crystal lattices, it is released during heating events, and being a gas, will eventually make it way to the atmosphere. A major portion of the terrestrial pool of argon has been produced since the Earth’s formation from the radioactive decay of the tiny amounts of the isotope potassium-40 (40-K = 0.012% of all potassium) found within potassium bearing minerals. Measuring the production of argon-40 (40-Ar) from the decay of 40-K is one of the more commonly used methods for determining the Earth’s age (potassium-argon dating).
Argon (Ar) is the most abundant of the inert Noble gases within our atmosphere. It is a colorless, odorless, non-reactive gas that is the 3rd to 4th most abundant gas (Ar vs. H2O) in the air that we breath and the atmospheres of caves.
Although atmospheric argon is ultimately derived from the decay of radioactive 40-K, it is not measurably radioactive itself and consists of just 3 stable isotopes.
The adjacent nuclear reaction is the portion of the decay of Uranium 238 into Lead 206 that produces Radon 222 gas.
Rn 222 is the most abundant of the radon isotopes and the only isotope that the name “radon” formally applies to, although in practice they are all usually lumped together under this name.Now for a bit of educated speculation on my part. To the best of my knowledge, the following theory on argon’s ability to accumulate in cave air is untested. Be that as it may…
Diffusion Coefficient Graph
Diffusion Coefficients
Argon (Ar) is the most abundant of the heavier-than-air gasses found in cave atmospheres, and like CO2 and radon (Rn), it should have the potential to accumulate in areas with restricted air flow.
Argon’s density is among the highest and it’s rate of diffusion the lowest of all the principal gases in cave atmospheres, so it seems logical that it may have the tendency to accumulate.
Argon has a very low rate of diffusion, which is the primary factor for predicting gas accumulation. Gaseous rates of diffusion are compared by their respective diffusion coefficients. Assuming an average cave temperature of 10C (50F), argon’s diffusion coefficient is 0.173 cm2/s, which is only 18% higher than that of CO2 (0.147 cm2/s). Overall, it is the third lowest after radon (0.104 cm2/s) amongst common cave-air gases.
Argon is also one of the densest of the atmospheric gases. It has a density of 1.78 kg/m3 , which is just 10% less than that of carbon dioxide (1.98 kg/m3). Carbon dioxide readily accumulates and often displays pronounced seasonal variations in cave air concentration and distribution.Under the right conditions, therefore, argon (Ar) should accumulate in much the same way as does it’s far less abundant noble gas cousin, radon (Rn) and/or carbon dioxide (CO2).
Under which conditions that might be, however, has not been widely studied or recognized.; it should be, in my opinion.
Why? Because argon is the most abundant of the non-reactive noble gases present in cave atmospheres. Many of the noble gases (argon included) have been used as geochemical tracers of both aqueous and gaseous solutions in non-spelean environments, and these types of solutions are key participants in the formation and development of karst and caves.
Argon's Spectral Lines
A few speculations as to the conditions under which argon (Ar) enrichment might occur would perhaps be germane at this juncture.
- The potential for accumulation is likely to be highest in low spots with restricted ventilation, as this is observed with the other heavy gasses, and there is no reason to suggest that this is not the case with Argon, as well.
- High gaseous concentrations of 40-Ar might be reasonable to expected in caves when there is potassium (K) rich bedrock either stratigraphically below the cave or nearby.
- Gaseous enrichment is also possible where cave networks intersect an argon saturated water table or where hypogene groundwaters such as thermal or other solute/gas enriched solutions are known or suspected to be involved in the speleogenetic process.
Hourglass Lake in Jewel Cave - Madison Aquifer Water Table
The Use of Argon Isotopes in Groundwater Dating & Source Area Characterization
Argon’s chemical and isotopic properties make it an ideal tracer of Earth processes. There is a considerable amount of information that argon isotopic compositions and concentrations can provide if the time and effort are put forth to acquire and assess them.
Noble gases like argon (Ar), helium (He), neon, (Ne), krypton (Kr) and xenon (Xe) are chemically inert and many of the complications that are common in interpreting isotopic data in other methodologies are absent when working with such tracers.
The patterns of noble gas isotopic variation are directly related to the source area and formations from which the gas originated and the path that it has taken prior to it’s final destination. This is true of both the anhydrous gases contained in the pore spaces of formations and those that are dissolved in groundwater. Although virtually all of the geochemical research done on argon isotopic variation has been done on groundwater, it should also be applicable to the argon that finds its way into cave air from formation gas sources.
The patterns of Helium (He) and Argon (Ar) isotopic variation have been most widely studied, but Neon (Ne) Xenon (Xe) and Krypton (Kr) have more recently been looked at, as well. Argon’s abundance, however, makes it far easier to evaluated than most of the other noble gases.
Argon gas has about the same solubility in water as oxygen and thus can be readily dissolved in aqueous solutions. When surface precipitation enters the groundwater regime, it is generally saturated with argon and takes on its atmospheric isotopic signature.
The natural variation in Argon’s three stable isotopes has been used to determine the paleo-recharge temperature of groundwater. The dependence of argon’s solubility on temperature makes it possible to determine the recharge temperature of recent and paleo-groundwater by simply evaluating the total dissolved argon gas content and comparing it against it’s solubility limits as a function of temperature.
The assumption in this method is that the water does not pick up any additional argon along the flow path. Often enough, however, there are potassium mineral bearing lithologies within the aquifer. When there is dilution from additional argon, it is most likely to be due to radiogenic 40-Ar produced from reactions with these potassium bearing minerals within the rock matrix along the flow path (see below). If the flow path for groundwater is dominantly through carbonates, however, argon dilution is less likely to be as prevalent, and in karst aquifers, flow paths restricted to carbonates is generally the case.
PictureThe ratios of radiogenic 37-Ar, 39-Ar, and 40-Ar concentrations in groundwater can also provide information about the relative ages of the different groundwater sources involved in cave formation. These ratios should also be reflected in cave atmospheric values when exchange with outside air is at a minimum and lithologic and groundwater contributions to cave air are the greatest (during the cool season)
- The half life of 37-Ar is only 35 days and that for 39-Ar is 269 years, so the later is used in most age estimations.
- Age dating using the 39-Ar abundance is possible out to about 2000 years or so (~7 half lives).
- Both 37-Ar and 39-Ar are primarily produced in the atmosphere from cosmic rays by the reactions shown in the adjacent diagram….but….
- They can also be produced, under the right conditions, from in-situ reactions within the rock matrix through which the groundwater flows.
- The presence of uranium or thorium in the rock matrix is the primary cause of this type of interference.
- In general, only minimal interference from the in-situ reactions listed above occurs for the 39-Ar reaction, whereas the 37-Ar reaction is often masked by interference and this is most particularly the case in Ca rich matrices (like limestone!). This interference, however, can yield some fairly useful information (see next section).
The n(40-Ar)/n(36-Ar) Ratio in Groundwater Dating
- The n(40Ar)/n(36Ar) ratio has also been used to aid in groundwater dating. This ratio has a constant value in the atmosphere as a whole of 295.5, but in groundwater, higher ratios are often the case.
- Most aquifers contain some potassium-bearing minerals, and the 40-K within these rocks decays and over time may cause this ratio to increase. The isotopic ratio can be used to age date very old groundwater, as long as the relative production rate of 40-Ar and releas within the aquifer can be estimated. This rate can be deduced from measuring the levels of 37-Ar within the groundwater (see next section)
- The groundwater n(40Ar)/n(36Ar) ratio of a given source can be substantially compromised by the addition of radiogenic 40-Ar from nearby potassium rich lithologies.
- This source of contamination comes from rocks that are not within the aquifer itself but which are producing argon that has migrated into the system from nearby areas.
The usage of 37-Ar levels as an aid in interpreting Argon Isotope patterns
- Because of the short half life of 37-Ar, groundwater has virtually no cosmogenically produced 37-Ar present. However, subsurface production of this isotope is common from rock matrix reactions involving the trace uranium and thorium found in many minerals.
- By measuring the amount of 37-Ar present, the production rate within an aquifer or formation can be assessed, the neutron flux level can be thereby deduced, and the efficiency of mineral to water transfer of Argon estimated. All of these values are essential for defining how the other radon isotopes may be used and interpreted in hydrologic and spelean applications.
The usage of 39-Ar in Groundwater Dating
- 39-Ar has been mainly used in dating groundwater in conjunction with the usage of other isotopic methods. With a half life of 269 years, age determinations using 39-Ar overlap with the high end of the tritium range (3-H), and the low end of the carbon-14 (14-C) range. The 39-Ar method is most useful for dating young (~40 to ~2000 years B.P.) groundwater, and this is generally the age range most applicable to speleogenetic processes, as well.
Synopsis
Even though argon is one of the four most abundant components of cave air, it’s variability in abundance and distribution within spelean environments is essentially an unknown. To the best of my ability to ascertain, it has not been studied in cave environments to date. Argon is a heavier than air gas that should have the the potential to locally accumulate, very much like CO2 and it’s radioactive noble gas cousin radon (Rn). This gas should therefore be subject to variability in concentration and distribution due to oscillations in cave air dynamics.
Like radon, argon bears a radiogenic isotopic signature and has be used successfully as a tracer of geochemical processes within lithologic and hydrologic systems. By inference , argon should also be useful as a tracer of these processes as they are uniquely expressed in cave environments.
A systematic look at argon abundances and isotopic patterns within cave air and waters would seem overdue, but as a geochemist who is also a caver, that’s just might be my personal bias shining through. So be it.
“the eternal recurrence of the same”
Also sprach Zarathustra – Friedrich Nietzsche
Click on one of the above links for more information on the nature and distribution of these heavy gas components of cave atmospheres.
Current Research Relating to Black Hills Cave Barometry and Atmospheric Geochemistry
The question of the basic air flow mechanisms in barometric caves and determining the full size of both the Wind and Jewel Cave systems are the main aspects of an ongoing research project from the Cave- and Subway-Climatology working group at the Ruhr-University of Bochum in Germany. This project is being spear-headed by Andreas Pflitsch of the Ruhr-University and his graduate students along with National Park Service personnel at both caves. Numerous Pahasapa grotto members have also provided help and support for the project, and barometric monitoring has recently been expanded to other caves within the southern hills region.
https://ojs.zrc-sazu.si/carsologica/article/view/75
Another ongoing research project involves an assessment of the seasonal concentrations and distribution of radon, carbon dioxide ,water vapor and temperature within Black Hills Caves. This study is currently in its infancy and only a few caves have been evaluated so far, but the goal is to expand the testing and monitoring to a representative population of Hills caves so as to establish baseline atmospheric concentrations for these gases and atmospheric parameters.
One aspect of this study is the determination of the seasonal patterns of CO2 concentration in both barometric and non-barometric caves in the area and to see whether CO2 concentrations may be effecting and/or limiting the speleogenetic process therein. Black hills caves are generally wet but sparsely decorated on the average, which is unusual, considering their generally accepted age. One possibility is that this paucity may be due to high ambient CO2 concentrations above 4000 ppm, which has been suggested as an upper limit beyond which speleothem precipitation may not be able to occur. Defining this atmospheric parameter may help to answer this question.
Another planned aspect of this study is to determine the seasonal and average concentrations of radon within our caves. This is needed both to establish an exposure baseline for occasional entrants such as cavers, and to see whether bats intrinsically avoid high radon areas within our area, as has been observed/suggested in some Mexican caves. https://www.researchgate.net/publication/257611454_Indoor_radon_concentration_levels_in_Mexican_caves_using_nuclear_track_methodology_and_the_relationship_with_living_habits_of_the_bats